A MODERN UNDERSTANDING OF POLYETHYLENETEREPHTHALATE THE DEGRADATION PROCESSES
DOI:
https://doi.org/10.15421/jchemtech.v31i3.285240Keywords:
polyethylene terephthalate; hydrolytic degradation; thermal degradation; thermo-oxidative degradation; thermomechanical degradation; photodegradation.Abstract
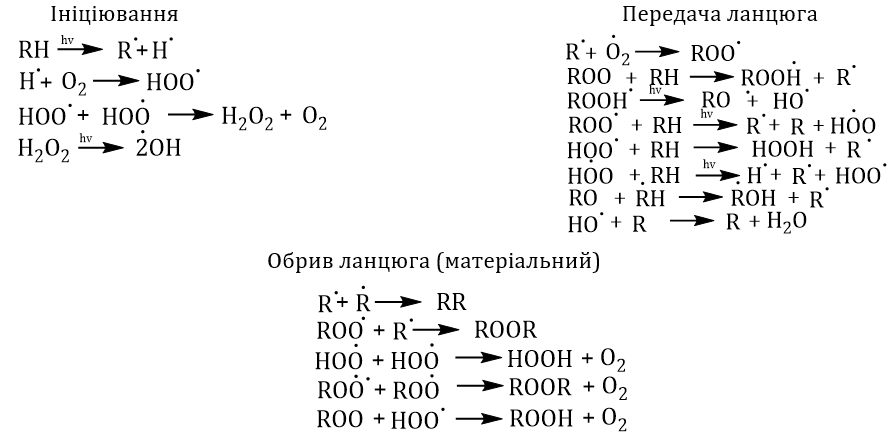
This work provides information about the current understanding of polyethyleneterephthalate destruction. It presents overall concepts about the mechanisms of hydrolytic, thermal, thermo-oxidative, thermomechanical and photo- destruction of polyethyleneterephthalate. By analyzing a wide range of literature sources, it was found that the degradation of polyethylene terephthalate occurs by many mechanisms and almost always causes an autocatalytic process of its degradation (hydrolysis) with the formation of polymeric and/or oligomeric derivatives with hydroxyl, carboxyl and vinyl ester groups, ethylene, acetaldehyde and cyclic compounds. It has been shown that the initiation temperature of hydrolytic degradation is 100–120 ˚C and the rate of hydrolytic degradation is ~10000 time faster than its thermal and thermo-oxidative destruction. It was determined that during thermal degradation, the PET chain breaks by a mechanism that differs from the mechanism of its hydrolytic degradation and is accompanied by the formation of PET derivatives with different end groups. However, without oxygen, thermal degradation is 3 times faster than in its presence. All products formed during thermo mechanical degradation are chromatophores, leading to color changes and reducing the colorability of PET recycled products.
Photodestruction of PET is based on the mechanism of the Norrish photochemical reaction and is divided into 2 types – Norrish type 1 and Norrish type 2.
References
Saleh, H. E.-D. (Ed.). (2012). Polyester. InTechOpen. doi: 10.5772/2748
Mittermeier, C., Lion, A. (2020).Challenges in the experimental investigation of the caloric and thermomechanical behaviour of semi-crystalline polymers, Polymer Testing, Vol. 81, (106252), https://doi.org/10.1016/j.polymertesting.2019.106252
Chang, T. M. (1970). Kinetics of thermally induced solid state polycondensation of poly(ethylene terephthalate). Polym Eng Sci, 10, 364–368. https://doi.org/10.1002/pen.760100610
Myren, T.H.T.; Stinson, T.A.; Mast, Z.J.; Huntzinger, C.G.; Luca, O.R. (2020,)Chemical and Electrochemical Recycling of End-Use Poly(ethylene terephthalate) (PET) Plastics in Batch, Microwave and Electrochemical Reactors. Molecules 2020, 25, 2742.
https://doi.org/10.3390/molecules25122742
Molnar, B., Ronkay, F. (2019). Effect of solid-state polycondensation on crystalline structure and mechanical properties of recycled polyethylene-terephthalate. Polym. Bull. 76, 2387–2398 https://doi.org/10.1007/s00289-018-2504-x
Barredo, A., Asueta, A., Amundarain, I., Leivar, J., Miguel-Fernández, R., Arnaiz, S., Epelde, E., López-Fonseca, R., Gutiérrez-Ortiz, H. (2023). Chemical recycling of monolayer PET tray waste by alkaline hydrolysis, Journal of Environmental Chemical Engineering, 11(3), 109823. https://doi.org/10.1016/j.jece.2023.109823.
Khemani, K.C. (2000). A novel approach for studying the thermal degradation, and for estimating the rate of acetaldehyde generation by the chain scission mechanism in ethylene glycol based polyesters and copolyesters, Polymer Degradation and Stability, 67(1), 91–99. https://doi.org/10.1016/S0141-3910(99)00097-X.
Yang, W., Liu, R., Li, C., Song, Y., Hu, C. (2021). Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid, Waste Management, 135, 267–274, https://doi.org/10.1016/j.wasman.2021.09.009.
Huang, J., Meng, H., Luo, X., Mu, X., Xu, W., Jin, L., Lai, B., (2022). Insights into the thermal degradation mechanisms of polyethylene terephthalate dimer using DFT method, Chemosphere, 291(2). https://doi.org/10.1016/j.chemosphere.2021.133112.
Ma, T., Wang, W., Wang, R. (2023) Thermal Degradation and Carbonization Mechanism of Fe−Based Metal−Organic Frameworks onto Flame−Retardant Polyethylene Terephthalate. Polymers, 15(1), 224
https://doi.org/10.3390/polym15010224
Chelliah A., Subramaniam M., Gupta, R., Gupta, A. (2017). Evaluation on the Thermo-Oxidative Degradation of PET using Prodegradant Additives, Indian Journal of Science and Technology, 10(6), 1–5. doi: 10.17485/ijst/2017/v10i6/111212
Romão, W., Franco, M., Corilo, Yu., Eberlin, M., Spinacé, M., De Paoli, M. (2009). Poly (ethylene terephthalate) thermo-mechanical and thermo-oxidative degradation mechanisms, Polymer Degradation and Stability, 94(10), 1849–1859. https://doi.org/10.1016/j.polymdegradstab.2009.05.017.
Colin, X., Tcharkhtchi A. (2013). Thermal degradation of polymers during their mechanical recycling. Recycling : Technological Systems, Management Practices and Environmental Impact, Nova Science, 978-162618283-7, 57–95.
Fechine, G. J. M.Souto-Maior, R. M., Rabello, M. S., (2002). Structural changes during photodegradation of poly(ethylene terephthalate), Journal of Materials Science, 37, 4979–4984. https://doi.org/10.1023/A:1021067027612
Pickett, J., Moore, J. (1993). Photodegradation of UV screeners, Polymer Degradation and Stability, 42(3), 231–244. https://doi.org/10.1016/0141-3910(93)90219-9.
Ding, L., Yu,X., Guo, X., Zhang, Ya., Ouyang, Zh, Liu, P., Zhang, C., Wang, T., Jia, H., Zhu, L. (2022). The photodegradation processes and mechanisms of polyvinyl chloride and polyethylene terephthalate microplastic in aquatic environments: Important role of clay minerals, Water Research, 208. https://doi.org/10.1016/j.watres.2021.117879.
Arhant, M., Gall, M.,Le Gac, P., Davies, P. (2019). Impact of hydrolytic degradation on mechanical properties of PET - Towards an understanding of microplastics formation, Polymer Degradation and Stability, 161, 175–182. https://doi.org/10.1016/j.polymdegradstab.2019.01.021.
Kawahara, Yu., Taiy, Yo, Wataru, T., Takeshi, K., Masaki, T. (2016). Alkaline Hydrolysis Kinetics of Poly(ethylene terephthalate) Fibers, Journal of Fiber Science and Technology, 72(1), 9–16. doi:10.2115/fiberst.72.1_9
Mendes, L. C., Pereira, P. S. C. (2012). Solid State Polymerization: Its Action on Thermal and Rheological Properties of PET/PC Reactive Blends. Polímeros, 23(3), 298-304. doi:10.4322/polimeros.2013.031
Ügdüler, S., Van Geem, K., Denolf, R., Roosen, M., Mys, N., Ragaert, K., De Meester, S. (2020). Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis, Green Chem., 16. http://dx.doi.org/10.1039/D0GC00894J
Shady, F., Konda, R., Basu, A., Domb, A. (2015). Molecular Weight Determination of Polyethylene Terephthalate, Poly(Ethylene Terephthalate) Based Blends, Composites and Nanocomposites, 2015, 143–165. https://doi.org/10.1016/B978-0-323-31306-3.00008-7.
Brenz, F., Linke, S., Simat, T.J. (2021). Linear and cyclic oligomers in PET, glycol-modified PET and Tritan™ used for food contact materials. Food Addit Contam Part A Chem Anal Control Expo Risk Assess., 38(1), 160–179. doi: 10.1080/19440049.2020.1828626.
Begley, H., Hollifield, H. (1990). Evaluation of polyethylene terephthalate cyclic trimer migration from microwave food packaging using temperature‐time profiles, Food Additives & Contaminants, 7(3), 339–346. doi: 10.1080/02652039009373898.
Xu, T., Qiu, K., Gao, H., Wu, G., Zhang, B. Zhao, Q., Zhang, Yu. (2021). Simultaneous determination of cyclic PET and PBT oligomers migrated from laminated steel cans for food, Food Control, 130, 108396. https://doi.org/10.1016/j.foodcont.2021.108396.
Tsochatzis, E.D., Lopes, J.A., Kappenstein, O., Tietz, T. Hoekstra, E. J. (2020). Quantification of PET cyclic and linear oligomers in teabags by a validated LC-MS method – In silico toxicity assessment and consumer’s exposure, Food Chemistry, 317, 126427. https://doi.org/10.1016/j.foodchem.2020.126427.
Tian, G., Yang, Zh., Zhang, W., Chen, Si-C., Li C., Wu, G. Wang, Yu-Zhong (2022) Integration of upcycling and closed-loop recycling through alternative cyclization–depolymerization, Green Chemistry, Vol. 22, Iss. 11, 4490-4497 http://dx.doi.org/10.1039/D2GC00853J
Cho, J.S., Youk, J.H., Jo, W.H., Ko, S.W., Ha, W.S. and Yoo, D.I., (2001). Cyclization Routes for Formation of Cyclic Oligomers in Poly(ethylene terephthalate), Macromolecular Chemistry and Physics, 202(7), 998–1003, https://doi.org/10.1002/1521-3935(20010401)202:7<998::AID-MACP998>3.0.CO;2-N
Ubeda, S., Aznar, M., Rosenmai, A. K., Vinggaard, A.M., Nerín C. (2020). Migration studies and toxicity evaluation of cyclic polyesters oligomers from food packaging adhesives, Food Chemistry, 311, 125918 https://doi.org/10.1016/j.foodchem.2019.125918.
Holland, B.J., Hay J.N. (2002). Analysis of comonomer content and cyclic oligomers of poly(ethylene terephthalate), Polymer, 43(6), 1797–1804. . https://doi.org/10.1016/S0032-3861(01)00773-X.
Djapovic, M., Milivojevic, D., Ilic-Tomic, T., Lješević, M., Nikolaivits, E., Topakas, E., Maslak, V., Nikodinovic-Runic, J. (2021). Synthesis and characterization of polyethylene terephthalate (PET) precursors and potential degradation products: Toxicity study and application in discovery of novel PETases, Chemosphere, 275. https://doi.org/10.1016/j.chemosphere.2021.130005.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

