INVESTIGATION OF THE ELECTROCHEMICAL BEHAVIOR OF CATHODES OF A RESERVE CHEMICAL CURRENT SOURCE
DOI:
https://doi.org/10.15421/jchemtech.v31i4.286037Keywords:
backup current sources; cathode; titanium; steel; lead; lead dioxide; voltammetry; galvanic coating; polarization measurements.Abstract
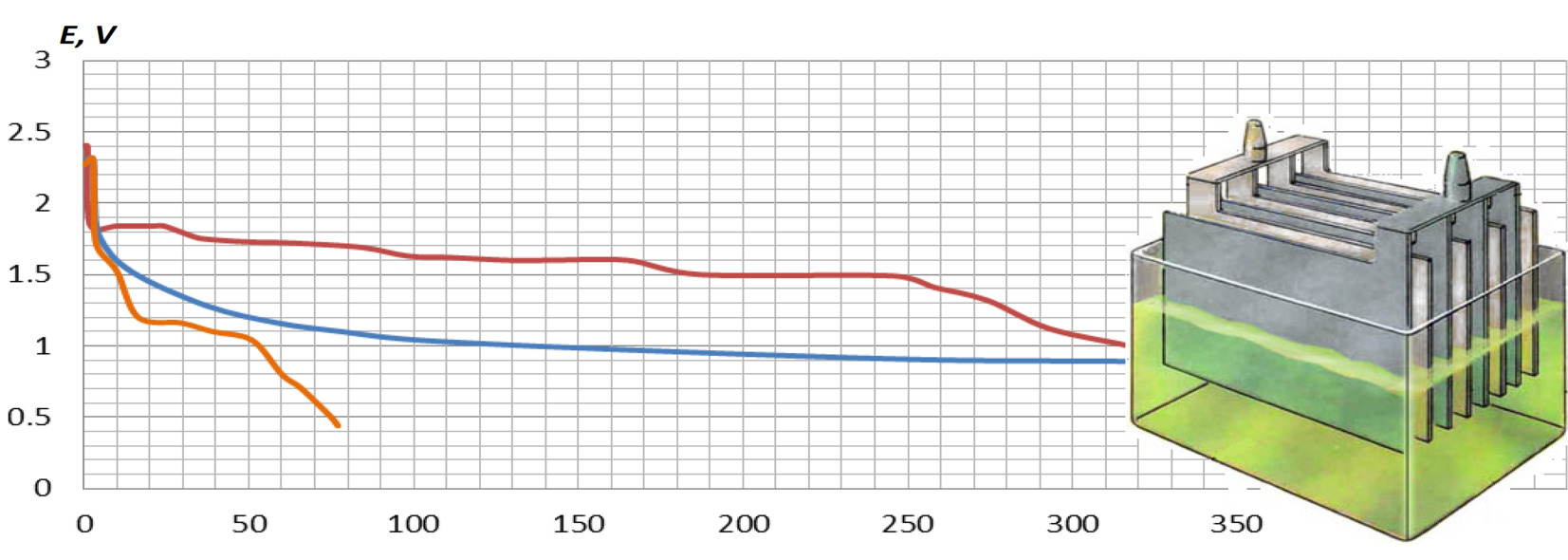
The aim of the work is the first stage in the development of a new ampoule backup chemical current source based on the lead dioxide reduction reaction, namely the selection of the cathode base material. The main objects of the study were cathodes made of titanium, steel, and lead with a galvanic coating of lead dioxide formed from a nitrate electrolyte using current density 1…2 A/dm2. To obtain a titanium cathode, the problem of surface passivation was solved using an initial symmetric cathode polarization for 30 seconds. To establish the dependence of the specific characteristics of the system on the material of the base, discharge curves were obtained and the dynamics of the discharge of different cathodes were established. The discharge curves were taken at a constant resistance of 7.3 Ω using a system with zinc anodes and perchloric acid as an electrolyte. The maximum stable discharge potential of the current source was found in titanium cathodes (1.8 V for 23 seconds). Moreover, when obtaining the polarization curves, the tendency of the steel cathode to peel off the coating and corrosion in the electrolyte was revealed, while the titanium cathode demonstrated stable current rates from cycle to cycle. Researching of the lead cathode showed low adhesion and/or conductivity between the formed powder coating and the base material. The study revealed that the optimal material for the base of the lead dioxide cathode for the backup current source, both from the point of view of chemical stability and discharge characteristics, was titanium. Therefore, it will be used in further studies of other components of the electrochemical system.
References
Ritchie, A., Bagshaw, N. (1996). Military Applications of Reserve Batteries. Philos. Trans. R. Soc., 354(1712), 1643–1652. https://doi.org/10.1098/rsta.1996.0070
Brinkert, K., Mandin, P. (2022). Fundamentals and future applications of electrochemical energy conversion in space. npj Microgravity. 8(52). https://doi.org/10.1038/s41526-022-00242-3
Lowy, D. A., Bence, M. (2020). Sea Water Activated Magnesium-Air Reserve Batteries: Calculation of Specific Energy and Energy Density for Various Cell Geometries. DRC Sustainable Future. 1(1), 1–6. https://doi.org/10.37281/DRCSF/1.1.1
Pourfarzad, H., Shabani-Nooshabadi, M., Ganjali, M. R., Olia, M. H. (2020). Inhibition of acid corrosion of glass ampoule in Pb/HBF4/PbO2 reserve batteries using nanobis [3(trimethoxysilyl)propyl]amine. J. Mol. Liq. 302, 112578. https://doi.org/10.1016/j.molliq.2020.112578
Moshkovskyi, M.S., Havryliuk, A.O., Kniazskyi, O.V., Liniucheva, O.V., Byk, M.V. (2022). [Analysis of chemical sources of current in russian samples of rocket and artillery weapons]. Collection of scientific works of the VI International scientific and practical conference "Chemical technology: science, economy and production", 64–74. (In Ukrainian).
Boloskhaan, C., Umbetalkaiyev, K. A., Mansurov, Z. A., Tulepov, M.І., Alipbaev, A.N. (2020). Obtaining and research of the physical and chemical properties the reserve current source on the basis of alumimum and zinc. The Journal of Almaty Technological University. 1(23), 89–93. (In Kazakh). https://www.vestnik-atu.kz/jour/article/view/260?locale=en_US
Sayeed, H., Nahian, S., Ratigul, H. (2019). Lead Acid Battery Monitoring and Charging System for Backup Generators. International Conference on Robotics, Electrical and Signal Processing Techniques (ICREST), https://doi.org/10.1109/ICREST.2019.8644475
Lutjering, G., Williams, J. (2007). Titanium. Springer Berlin, Heidelberg https://doi.org/10.1007/978-3-540-73036-1
Kosohin, О. V., Kushmyruk, A. І., Miroshnychenko, Y. S., Linyucheva O. V. (2012). Electrochemical properties of titanium-based catalytically active electrodes in perchloric acid. Mater Sci 48(4), 139–146. (In Ukrainian). https://doi.org/10.1007/s11003-012-9483-0
Shin, J., Kim J., Kim K., Ahn, H. (2002). Discharge characteristics of lead dioxide electrode prepared with cementation lead oxide. Metals and Materials International, 8, 417–422. https://doi.org/10.1007/BF03186116
Chen, Z, Xie, G., Pan, Z., Zhou, X. (2021). A novel Pb/PbO2 electrodes prepared by the method of thermal oxidation-electrochemical oxidation: Characteristic and electrocatalytic oxidation performance J. of All. and Comp. 8, 156834. https://doi.org/10.1016/j.jallcom.2020.156834
Guifeng, Y. (2022). China Patent №114678602A. Beijing, China.
Guohua, Z., Yonggang, Z., Yanzhu, L. (2009). China Patent №102043004B Beijing, China.
Muratova, O. M, Tulskii, H. H, Brovin, O. Y., Bayrachnyi, V. B. (2005) Ukraine Patent №10994U Kyiv, Ukraine.
Brown, H. (1985). Lead Oxide. Properties and Applications. Lisbon. International Lead Zinc Research Organization
Velichenko, A. B., Girenko, D. V., Danilov, F. I. (1996). Mechanism of lead dioxide electrodeposition. J. Electroanal. Chem. 405(2), 127–132. https://doi.org/10.1016/0022-0728(95)04401-9
Mykhaylenko, V. G., Antonov, A. V. (2014). A Study of the Electrodeposition of Lead Dioxide from Alkaline Baths – Deposition Process and Coating Quality. Electroplating and surface treatment 22(2). http://www.galvanotehnika.info/pdf/2014/2/gtech.14.2.(29-35).pdf
Banjo, N., Sasaki, T. T., Hono, K. (2022). Microstructural origin of adhesion and corrosion properties of Ti-based conversion coatings on A6063 alloy. Appl. Surf. Sci. 604, 154411. https://doi.org/10.1016/j.apsusc.2022.154411
Guohua, Z., Yonggang, Z., Yanzhu, L. (2010). China Patent № 102190351B Beijing, China.
Fuyue, Z., Li D., Rong, W., Yang, L., Zhang, Y. (2021). Study of Microscale Meniscus Confined Electrodeposition Based on COMSOL. Micromachines 12, 1591. https://doi.org/10.3390/mi12121591
Ajayi-Majebi, J., Abioye, O. P., Fayomi, O. S. I., Oyedepo, S. O., Ayara, W. A. (2020). A concise overview on optimization and modelling parameter cases in electrodeposition and composite coating technology IOP. Conf. Ser.: Mater. Sci. Eng. 1107, 012081. https://doi.org/10.1088/1757-899X/1107/1/012081
Fernandez, A. P. R., Perigo, E. A., Faria, R. N. (2022). Simulation of galvanostatic charge-discharge curves of carbon-based symmetrical electrochemical supercapacitor with organic electrolyte employing potential dependent capacitance and time domain analytical expressions. Journal of Energy Storage. 51, 104471. https://doi.org/10.1016/j.est.2022.104471
Jadhav, N., Gelling, V. (2019). The Use of Localized Electrochemical Techniques for Corrosion Studies. J. Electrochem. Soc. 166 (11). https://doi.org/10.1149/2.0541911jes
Quej-Ake, L.M., Contreras, A. (2018). Electrochemical study on the corrosion rate of X52 steel exposed to different soils. Anti-Corrosion Methods and Materials. 65(1). 97–106. https://doi.org/10.1108/ACMM-12-2016-1737
Shao, D., Wang, Z., Zhang, C., Li, W., Xu, H., Tan, G., Yan W. (2022). Embedding wasted hairs in Ti/PbO2 anode for efficient and sustainable electrochemical oxidation of organic wastewater. Chin. Chem. Lett. 33(3). 1288–1292. https://doi.org/10.1016/j.cclet.2021.07.061
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

