STRUCTURE, OPTICAL PROPERTIES AND PHOTOCATALYTIC ACTIVITY OF UNDOPED, Y2O3-DOPED ZnO NANOCOMPOSITES
DOI:
https://doi.org/10.15421/jchemtech.v32i1.286092Keywords:
ZnO-Y2O3 nanopowders, zinc oxide, yttrium oxide,, photocatalysis, degradationAbstract
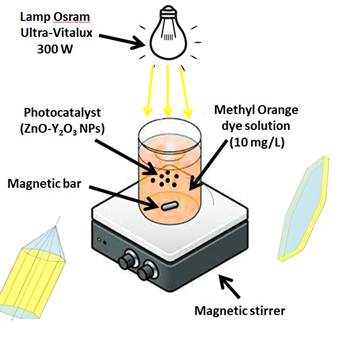
Y-doped ZnO nanocomposites with different content of Y2O3 (1–5 %) were obtained by the Pechini method from their nitrate solutions. The solutions of Zn2+ and Y3+ nitrates were preliminary obtained by dissolving of zinc and yttrium oxides with a content of the main component of 99.99% in nitric acid. The influence of yttrium doping the on the microstructure, morphology, optical properties and photocatalytic activity of the ZnO nanopowders were examined. The properties of the nanopowders were studied by using X-ray phase analysis, scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy. The samples were subjected to X-ray powder diffraction using a DRON-3 diffractometer (Cu-Ka radiation) at room temperature. X-ray phase analysis confirms the formation of single phase of Y2O3-doped ZnO powders on diffractograms. According to SEM results, the powders characterized a conglomerate structure. The undoped ZnO has an average particle size of 43 nm, while the average particle size of Y3+-doped ZnO ranges from 63 to 79 nm. It was established that the morphology of powder particles primarily depends on the content of Y3+ in the material. Raman scattering measurements indicate that ZnO samples doped with Y2O3 have an intense and clearly expressed A1TO mode, which may be related to the deformation of the powder granules. In the photoluminescence spectra of ZnO powders, with increasing Y2O3 concentration, bands at 400 nm are observed due to the appearance of impurities that cause of interstitial zinc and zinc vacancy defects and their broadening with a shift to the long-wave region. Photocatalytic properties of ZnO powders doped with yttrium oxide were investigated using Methyl Orange as a model dye under Osram Ultra-Vitalux lamp (300 W) irradiation. A present result indicates that the obtained powders are potential candidate for the practical application in photocatalysis.
References
Gupta, J., Hassan, P., Barick, K. (2021). Structural, photoluminescence, and photocatalytic properties of Mn and Eu co-doped ZnO nanoparticles. Mater. Today: Proc., 42(2), 926–931. https://doi.org/10.1016/j.matpr.2020.11.837
Kumawat A., Chattopadhyay S., Verma R. K., Misra K.P. (2022). Eu doped ZnO nanoparticles with strong potential of thermal sensing and bioimaging. Mater. Lett., 308(8), 131–221. https://doi.org/10.1016/j.matlet.2021.131221
Gnanaprakasam A., Sivakumar V., Thirumarimurugan M. (2018). Investigation of Photocatalytic Activity of Nd-Doped ZnO Nanoparticles Using Brilliant Green Dye:Synthesis and Characterization. Iran. J. Chem. Chem. Eng., 37, 61 71. https://doi.org/10.30492/ijcce.2018.30658
Lili H., Shenmao Z., Yuequan D. (2019). Preparation of rare earth Nd-doped ZnO nano-materials and its degradation of Rhodamine B. Inorganic Chemicals Industry, 51(8), 88–92. https://doi.org/ 10.11962/1006-4990.2019-0139
Upadhyay P. K., Sharma N., Sharma S., Sharma R. (2021). Photo and thermoluminescence of Eu doped ZnO nanophosphors. J. Mater. Sci.: Mater. Electron., 32, 17080–17093.
https://doi.org/10.1007/s10854-021-06043-w
Gong X., Jiang H., Cao M., Rao Z., Zhao X. Vomiero A. (2021). Eu-doped ZnO quantum dots with solid-state fluorescence and dual emission for high-performance luminescent solar concentrators. Mater. Chem. Front., 5, 4746–4755.
https://doi.org/10.1039/D1QM00178G
Khataee A., Karimi A., Zarei M., Joo S. (2020). Eu-doped ZnO nanoparticles: Sonochemical synthesis, characterization, and sonocatalytic application. Ultrason. Sonochem., 67, 102822. https://doi.org/10.1016/j.ultsonch.2015.03.016
Satpal, S.B., Athawale, A.A. (2018). Synthesis of ZnO and Nd doped ZnO polyscales for removal of rhodamine 6G dye under UV light irradiation. Mater. Res.Express, 5, 085501. https://doi.org/10.1088/2053-1591/AAD26C
Sin, J. C., Lam, S. M. (2018). One-dimensional ZnO nanorods doped with neodymium for enhanced resorcinol degradation under sunlight irradiationvol. Chem. Eng. Commun., 205, 311–324. https://doi.org/ 10.1080/00986445.2017.1387855. 24.
Samanta, A., Goswami, M. N., Mahapatra, P. K. (2019). Influence of Nd3+ doping in ZnO nanoparticles to enhance the optical and photocatalytic activity. Mater. Res.Express, 6, 065031. https://doi.org/ 10.1088/2053-1591/ab0c25
Kayani, Z. N., Amir, B., Riaz, S., Naseem, S. (2020). Antibacterial, magnetic, optical and dielectric analysis of novel La2O3 doped ZnO thin films. Opt. Mater., 109, 110–287. https://doi.org /10.1016/j.optmat.2020.110287
Li, C., Hun, R., Qin, L., Ding, R., Li, X., Wu, H. (2013). Enhanced photocatalytic activity of ZnO/La2O3 composite modified by potassium for phenol degradation. Mater. Lett., 113, 190–194. https://doi.org /10.1016/j.matlet.2013.09.050
Kayani, Z. N., Benish, A., Riaz, S., Naseem, S. (2020). Antibacterial, magnetic, optical and dielectric analysis of novel La2O3 doped ZnO thin films. Opt. Mater., 109, 110–287. https://doi.org /10.1016/j.optmat.2020.110287
Hsieh, P.-T., Chuang, R.-K., Chang, C.-Q., Wang, C.-M., Chang, S.-J. (2011). Optical and structural characteristics of yttrium doped ZnO films using sol-gel technology. J. Sol-Gel Sci. Technol., 58, 42–47. https://doi.org 10.1007/s10971-010-2352-0
AlAbdulaal, T.H., AlShadidi, M., Hussien, Mai S. A., Vanga, G., Bouzidi, A., Rafique, S., Algarni, H., Zahran, H.Y., Abdel-wahab, M.Sh., Yahia, I.S. (2021). Structural, morphological and optical bandgap analysis of multifunction applications of Y2O3-ZnO nanocomposites: Varistors and visible photocatalytic degradations of waste water. Research Square, https://doi.org/10.21203/rs.3.rs-391412/v1
Sanoop, P. K., Anas, S., Ananthakumar, S., Gunasekar, V., Saravanan, R., Ponnusami, V. (2016). Synthesis of yttrium doped nanocrystalline ZnO and its photocatalytic activity in methylene blue degradation. Arabian J. Chem., 9, 1618–1626. https://doi.org/10.1016/j.arabjc.2012.04.023
Divya, N. K., Pradyumnan, P. P. (2016). Solid state synthesis of erbium doped ZnO with excellent photocatalytic activity and enhanced visible light emission. Mater. Sci. Semicond. Process., 41, 428–435 https://doi.org/10.1016/j.mssp.2015.10.004
Zong Y., Li, Zhe, Wang, X., Ma, J., Men, Y. (2014). Synthesis and high photocatalytic activity of Eu-doped ZnO nanoparticles. Ceram. Int., 40, 10375–10382. https://doi.org/10.1016/j.ceramint.2014.02.123
Boltenkov, I.S., Kolobkova, E.V., Evstropiev, S.K. (2018). Synthesis and characterizationof transparent photocatalytic ZnO-Sm2O3 and ZnO-Er2O3 coatings. J. Photochem. Photobiol. A, 367, 458–464. https://doi.org/10.1016/j.jphotochem.2018.09.016
Evstropiev, S.K., Karavaeva, A.V., Dukelskii, K.V., Evstropyev, K.S., Nikonorov, N.V., Kolobkova, E.V. (2018). Transparent ZnO-Y2O3 coatings: bactericidal effect in the lighting and in the darkness. Ceram. Int., 44, 9091–9096. https://doi.org/10.1016/j.ceramint.2018.02.116
Chudinovych, O. V., Myroniuk, D. V., Myroniuk, L. A., Shyrokov, O. V., Danylenko, I. M. (2023). Structure, optical properties and photocatalytic activity of undoped, Nd2O3-doped ZnO nanocomposites. Funct. Mater., 30, 1–7. https://doi.org/10.15407/fm30.02.1
Mudavakkat, V. H., Noor-A-Alam, M., Bharathi, K. K., Bharathi, K., Kayani, A., Dissanayake, A., Ramana, Chintalapalle, V. (2011). Structure and AC conductivity of nanocrystalline Yttrium oxide thin films. Thin Solid Films, 519, 7947–7950. https://doi.org/10.1016/j.tsf.2011.04.222
Karpyna, V., Myroniuk, L., Myroniuk, D. (2023). Effect of Cobalt Doping on Structural, Optical, and Photocatalytic Properties of ZnO Nanostructures. Catal. Lett., 1–10. https://doi.org/10.1007/s10562-023-04493-x
Sidorenko, S.I., Barabash, R.I. (1997). [Modern X-ray diffraction analysis of real crystals], Naukova Dumka, Kiev (in Ukrainian).
Liu, Y., Wei, S., Gao, W. (2015). Ag/ZnO heterostructures and their photocatalytic activity undervisible light: Effect of reducing medium. J. Hazardous Mater., 287, 59–68. https://doi.org/10.1016/j.jhazmat.2014.12.045
Ahmad, M., Ahmed, E., Zhang, Y., Khalid, N. R., Xu, J., Ullah, M., Hong, Z. (2013). Preparation of highly efficient Al-doped ZnO photocatalyst by combustion synthesis. Current Appl. Phys., 13, 697–704. https:// doi: 10.1016/j.cap.2012.11.008
Zamiri, R., Lemos, A.F., Reblo, A., Ahangar, H. A., Ferreira, J.M.F. (2014). Effects of rare-earth (Er, La and Yb) doping on morphology andstructure properties of ZnO nanostructures prepared by wet chemical method. Ceram. Int., 40, 523–529. http://dx.doi.org/10.1016/j.ceramint.2013.06.034
Damen, T. C., Porto, S.P. S., Tell, B. (1966). Raman effect in zinc oxide. Phys. Rev., 142, 570. http://dx.doi.org/10.1103/physrev.142.570
Gruber, Th., Prinz, G.M., Kirchner, C., Kling, R., Reuss, F., Limmer, W., Waag, A. (2004). Influences of biaxial strains on the vibrational and exciton energies in ZnO. J. Appl. Phys., 96, 289–293. https://doi.org/10.1063/1.1755433
Schneider, L., Halm, S., Bacher, G., Roy, A., Kruis, F. E. (2006). Phys Status Solidi C., 3, 1014–1017. https://doi.org/10.1002/pssc.200564705
Yang J., Wang R., Yang L., Lang J., Wei M., Gao M., Liu X., Cao J., Li X., Yang N. (2011). Tunable deep-level emission in ZnO nanoparticles via yttrium doping. J. Alloy Compd., 509, 3606–3612. https://doi.org/10.1016/j.jallcom.2010.12.102
Ahmad, M., Ahmed, E., Zhang, Y., Khalid, N. R., Xu, J., Ullah, M., Hong, Z. (2013). Preparation of highly efficient Al-doped ZnO photocatalyst by combustion synthesis. Current Appl. Phys., 13, 697–704. https://doi: 10.1016/j.cap.2012.11.008
Kuriakose, S., Satpati, B., Mohapatra, S. (2014) Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys Chem Chem Phys., 16, 12741–12749. https://doi.org/10.1039/C4CP01315H
He, R., Hocking, R. K., Tsuzuki, T. (2012). Co-doped ZnO nanopowders: location of cobalt and reduction in photocatalytic activity, Mat. Chem. Phys. 132, 1035–1040. https://doi:10.1016/j.matchemphys.2011.12.061
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

