THE INFLUENCE OF FERROUS IONS AND THE ANODIC PROCESS ON THE LOCAL ELECTRODEPOSITION OF COPPER IN ELECTROCHEMICAL 3D-PRINTING SYSTEMS
DOI:
https://doi.org/10.15421/jchemtech.v31i4.286616Keywords:
electrochemical 3D-printing; sulfate electrolyte; throwing power; Fe3 ions; mass transfer.Abstract
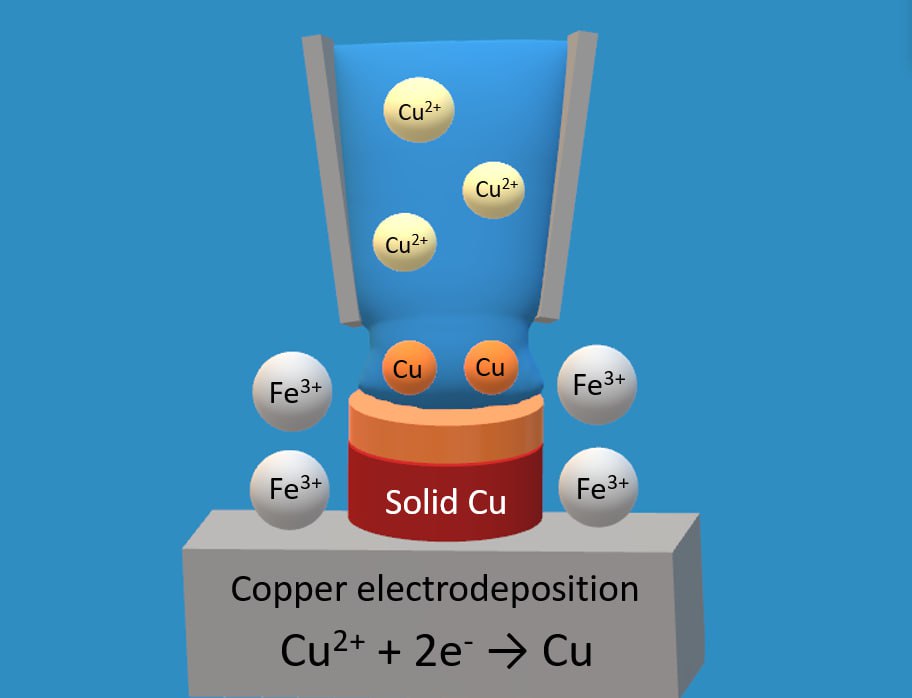
The effect of side cathode processes and anode materials on copper current efficiency in sulfate electrolyte and the accuracy of electrochemical 3D-printing have been studied. It has been established that the presence of 10 g/dm3 of Fe3+ ions in the electrolyte has different effects on the copper current output in cells with active copper and inert lead anodes. In the range of current densities of 5...7 A/dm2, the copper current efficiency in an electrochemical cell with a copper anode does not exceed 86...88%, while for a cell with a lead anode it is 96...97%. At a current density of
1 A/dm2, on the contrary, in a cell with a lead anode, the current efficiency decreases to 65%, and in a cell with a copper anode to 72%. The difference in the values of the current efficiency and the course of the obtained logarithmic dependences of the current efficiency on the current density may be related to the influence of the anodic oxygen emission process on the lead electrode on the side process of the reduction of Fe3+ ions. Oxidizing action of anodically produced oxygen in relation to the latter, as well as alkalization of the near-cathode layer leads to the formation of poorly soluble ferrous compounds and inhibition of their recovery in the region of high values of cathodic current densities. Thus, in a cell with an inert lead anode, the presence of Fe3+ ions in the solution leads to a decrease in copper efficiency in the range of low current densities. The modeling of the profile of long-term growth of a locally electrodeposited fragment of a copper precipitate in COMSOL Multiphysics software with consideration of the current efficiency and the results of polarization measurements has shown the following. This can contribute to increasing the localization of electrodeposition and the accuracy of electrochemical 3D-printing. The introduction of Fe3+ ions into the electrolyte leads to a decrease in the slope of the cathode polarization curve and, accordingly, leads to an increase in the throwing power of the electrolyte, which is more pronounced in a cell with a copper anode. A decrease in the thickness of the metal deposit in the range of low values of the current densities is reflected only by modeling the growth profile of the metal deposit for a cell with a lead inert anode.
Keywords: electrochemical 3D-printing; sulfate electrolyte; throwing power; Fe3+ ions; mass transfer.
References
Chen, X., Liu, X., Childs, P., Brandon, N., Wu, B. (2017). A low cost desktop electrochemical metal 3D printer. Advanced Materials Technologies, 2(10), 1700148.
https://doi.org/10.1002/admt.201700148
Behroozfar, A., Daryadel, S., Morsali, S. R., Moreno, S., Baniasadi, M., Bernal, R. A., Minary‐Jolandan, M. (2018). Microscale 3D printing of nanotwinned copper. Advanced materials, 30(4), 1705107. https://doi.org/10.1002/adma.201705107
Rafiee, M., Farahani, R. D., Therriault, D. (2020). Multi‐material 3D and 4D printing: a survey. Advanced Science, 7(12), 1902307. https://doi.org/10.1002/advs.201902307
Chen, X., Liu, X., Ouyang, M., Chen, J., Taiwo, O., Xia, Y., Childs, P.R.N., Brandon, N.P., Wu, B. (2019). Multi-metal 4D printing with a desktop electrochemical 3D printer. Scientific reports, 9(1), 3973. https://doi.org/10.1038/s41598-019-40774-5
Xu, J., Ren, W., Lian, Z., Yu, P., Yu, H. (2020). A review: development of the maskless localized electrochemical deposition technology. The International Journal of Advanced Manufacturing Technology, 110, 1731–1757. https://doi.org/10.1007/s00170-020-05799-5
Nakazawa, K., Yoshioka, M., Mizutani, Y., Ushiki, T., Iwata, F. (2020). Local electroplating deposition for free-standing micropillars using a bias-modulated scanning ion conductance microscope. Microsystem Technologies, 26, 1333–1342. https://doi.org/10.1007/s00542-019-04665-z
Vasyliev, G., Vorobyova, V., Uschapovskiy, D., Linucheva, O. (2022). Local electrochemical deposition of copper from sulfate solution. Journal of Electrochemical Science and Engineering, 12(3), 557–563. https://doi.org/10.5599/jese.1352
Vasyliev, G., Vorobyova, V., Uschapovskiy, D., Kotyk, M., Linyucheva, O. (2023). Influence of polarization curve slope on the accuracy of local copper electrodeposition from sulphate electrolyte: Original scientific paper. Journal of Electrochemical Science and Engineering. https://doi.org/10.5599/jese.1899
Brant, A. M., Sundaram, M. M., Kamaraj, A. B. (2015). Finite element simulation of localized electrochemical deposition for maskless electrochemical additive manufacturing. Journal of Manufacturing Science and Engineering, 137(1), 011018. https://doi.org/10.1115/1.4028198
Sundaram, M. M., Kamaraj, A. B., Kumar, V. S. (2015). Mask-less electrochemical additive manufacturing: a feasibility study. Journal of Manufacturing Science and Engineering, 137(2), 021006. https://doi.org/10.1115/1.4029022
Haba, T., Itabashi, T., Akahoshi, H., Sano, A., Kobayashi, K., Miyazaki, H. (2002). Electrochemical and simulative studies of trench filling mechanisms in the copper damascene electroplating process. Materials Transactions, 43(7), 1593–1598. https://www.jstage.jst.go.jp/article/matertrans/43/7/43_7_1593/_pdf
Wang, C., Zhang, J., Yang, P., An, M. (2013). Electrochemical behaviors of Janus Green B in through-hole copper electroplating: An insight by experiment and density functional theory calculation using Safranine T as a comparison. Electrochimica Acta, 92, 356–364. https://doi.org/10.1016/j.electacta.2013.01.064
Ehsani, A., Yazıcı, E. Y., Deveci, H., Erdemir, F. (2012). The Influence of Impurity Ions on The Electrowinning of Copper from Waste PCBs Leaching Solutions. In Proceedings of the XII International Mineral Processing Symposium, 443–449. doi: 10.13140/RG.2.1.2701.5200
Dew, D. W., Phillips, C. V. (1985). The effect of Fe (II) and Fe (III) on the efficiency of copper electrowinning from dilute acid Cu (II) sulphate solutions with the chemelec cell: Part I. Cathodic and anodic polarisation studies. Hydrometallurgy, 14(3), 331–349. https://doi.org/10.1016/0304-386X(85)90043-X
Das, S. C., Krishna, P. G. (1996). Effect of Fe (III) during copper electrowinning at higher current density. International journal of mineral processing, 46(1-2), 91–105. https://doi.org/10.1016/0301-7516(95)00056-9
Huyen, P. T., Dang, T. D., Tung, M. T., Huyen, N. T., Green, T. A., Roy, S. (2016). Electrochemical copper recovery from galvanic sludge. Hydrometallurgy, 164, 295–303. http://dx.doi.org/10.1016/j.hydromet.2016.06.028
Yang, W., Wang, Y., Li, B., Wei, Y., Jiang, X. (2023). Effect of Fe3+ on electrowinning of copper by CE. Minerals Engineering, 191, 107942. https://doi.org/10.1016/j.mineng.2022.107942
Cooper, W. C. (1985). Advances and future prospects in copper electrowinning. Journal of applied electrochemistry, 15, 789–805. https://doi.org/10.1007/BF00614357
«Electrode growth next to an insulator» https://www.comsol.com/model/electrode-growth-next-to-an-insulator-10212
Ushchapovskyi, D. Yu., Vorobyova, V. I., Plivak, O. A., Motronyuk, T. I., Vasyliev, G. S. (2022). Limitations of copper nitrate electrolyte for fast electrochemical 3D-printing. Visnyk Cherkaskogo derzhavnogo tehnologichnogo universitetu. Technical science, (4), 77–87. https://doi.org/10.24025/2306-4412.4.2022.265832
Vasyliev, G. S., Ushchapovskyi, D. Yu., Vorobyova, V. I., Motronyuk, T. I., Linucheva, O. V. (2021). Development of additive technology - improvement of electrochemical 3d printing system. In Electrochemistry today: achievements, problems and prospects. Kyiv: МPBP Gordon (in Ukrainian) http://ionc.com.ua/PDF/Book-IX-EX-2021.pdf
Ushchapovskiy D.Yu. The copper electrowinning process intensification in complex processing of oxide ore with obtaining high profitable products (PhD dissertation). (in Ukrainian) https://ela.kpi.ua/bitstream/123456789/19401/1/Ushchapovskiy_diss.pdf
Rigby, G. D., Grazier, P. E., Stuart, A. D., Smithson, E. P. (2001). Gas bubble induced mixing in electrowinning baths. Chemical engineering science, 56(21-22), 6329–6336. https://doi.org/10.1016/S0009-2509(01)00233-0
Hreiz, R., Abdelouahed, L., Fünfschilling, D., Lapicque, F. (2015). Electrogenerated bubbles induced convection in narrow vertical cells: A review. Сhemical engineering research and design, 100, 268–281. http://dx.doi.org/10.1016/j.cherd.2015.05.035
Slesinski, A., Sroka, S., Fic, K., Frackowiak, E., Menzel, J. (2022). Operando Monitoring of Local pH Value Changes at the Carbon Electrode Surface in Neutral Sulfate-Based Aqueous Electrochemical Capacitors. ACS Applied Materials & Interfaces, 14(33), 37782–37792. https://doi.org/10.1021/acsami.2c09920
Bollella, P., Melman, A., Katz, E. (2020). Electrochemically Generated Interfacial pH Change: Application to Signal‐Triggered Molecule Release. ChemElectroChem, 7(16), 3386–3403. https://doi.org/10.1002/celc.202000615
Vasyliev, G. S., Ushchapovskyi, D. Yu., Vorobyova, V. I., Linucheva, O. V. (2021). Modelling approach in the development of electrochemical 3D-printing systems. KPI Science News, 2, 97–105. https://doi.org/10.20535/kpisn.2021.2.233716
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

