N-SUBSTITUTED PYRIDINE SALTS WITH PHTHALIMIDE-N-OXYL ANION
DOI:
https://doi.org/10.15421/jchemtech.v31i3.286682Keywords:
pyridine salt derivatives; nicotinamide; N-hydroxyphthalimide; phthalimide-N-oxyl salts; N-oxyphthalimide anion.Abstract
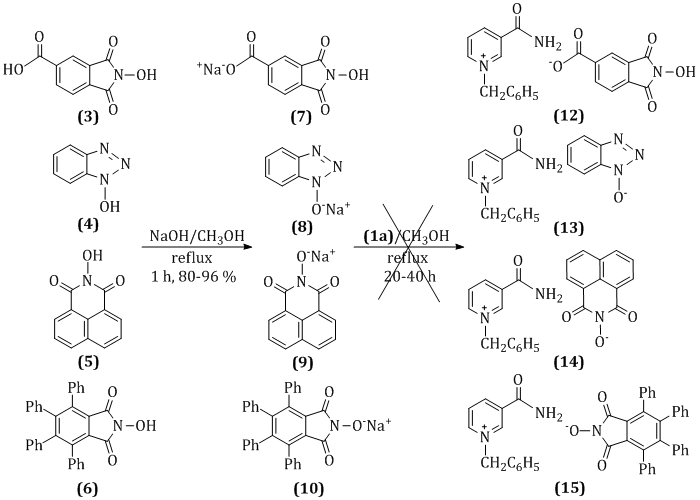
Currently, various biological activities of pyridine derivatives are widely studied, the type and strength of which depends on the presence of a substituent in the molecule. The aromatic pyridine ring plays an important role in the metabolism of a living organism. It is an oxidative system, cleaving the hydride in nicotinamide adenine dinucleotide (NAD+) – a component of the dehydrogenase enzyme. Derivatives of pyridine salts are part of a variety of medicines that are used for the prevention, diagnosis and treatment of diseases of the human body as well. Therefore, we synthesized new water-soluble derivatives of N-benzylpyridinium 3-carboxamide phthalimide-N-oxyl, N-propylpyridinium 3-carboxamide phthalimide-N-oxyl and N-methylpyridinium 3-carboxamide phthalimide-N-oxyl with a pyridine ring. The reactions occurred during the interaction of the corresponding N-substituted pyridine salts of nicotinamide with various phthalimide-N-oxyl salts. Also within the framework of the work, the method of joining N-oxyphthalimide anion to N-substituted pyridine molecule was investigated, selected and developed.
References
Nesterova, Ye. Yu., Нryshсhenko, Н. O. (2013). [The main directions of development of the chemistry of 1.4-dihydropyridines – a review of the literature]. Bulletin of Dnepropetrovsk University. Series «Chemistry», 21(19), 61–79. (in Russian). https://doi.org/10.15421/081308
Yedase, G. S., Venugopal, S., Arya, P., Yatham, V. R. (2022). Catalyst-free Hantzsch Ester-mediated Organic Transformations Driven by Visible light. Asian Journal of Organic Chemistry. 11(10). https://doi.org/10.1002/ajoc.202200478
Sadri, Z., Behbahani, F. K. (2020). Synthesis of Spiro1.4-Dihydropyridines: A Review. Current Organic Synthesis, 17(5), 324–343. https://doi.org/10.2174/1570179417666200415150027
Soma, M., Yamagishi, А. (1970). Photo-induced electron transfer from organic crystals to biologically interesting substances Biochimica et Biophysica Acta (BBA). Bioenergetics. 205, 183–189. https://doi.org/10.1016/0005-2728(70)90248-3
Han, B., Liu, Q., Liu, Zh., Mu, R., Zhang, W., Liu, Zh.-Li, Yu, W. (2005). A Metal-Free Catalytic Aerobic Aromatization of Hantzsch 1.4-Dihydropyridines by N-Hydroxyphthalimide. Journal of Organic Chemistry, 15, 2333–2334.
https://doi.org/10.1055/s-2005-872267
Ma, R., Chen, W., Wang, L., Yi, X., Xiao, Y., Gao, X., Zhang, J., Tang, X., Yang, Ch., Meng, X., Zheng, A., Xiao, F.-Sh. (2019). N-Oxyl Radicals Trapped on Zeolite Surface Accelerate Photocatalysis. ACS Catalysis, 9(11), 10448–10453.
https://doi.org/10.1021/acscatal.9b03737
Mancini, M. Di B., Gelsomino, A. D., Stefano, S. Di, Frateloreto, F., Lapi, A., Lanzalunga, O., Olivo, G., Sajeva, S. (2021). Change of Selectivity in C-H Functionalization Promoted by Nonheme Iron(IV)-oxo Complexes by the Effect of the N-hydroxyphthalimide HAT Mediator. ACS Omega, 6(40), 26428–26438. https://doi.org/10.1021/acsomega.1c03679
Zhu, Zh., Zhang, Q., Xie, D., Liu, H., Wang, H., Shi, L., Chen, Ch. (2022). Photo- and Solvent-Mediated Production of the Highly Reactive N-Oxyl Radical and Its Efficient Catalytic Oxidation of Hydrocarbons at Ambient Temperature. ACS Sustainable Chemistry. Engineering, 10(41), 13765–13774. https://doi.org/10.1021/acssuschemeng.2c03985
Kushch, O. V., Hordieieva, I. O., Novikova, K. V., Litvinov, Y. E., Kompanets, M. O., Shendrik, A. N., Opeida, I. A. (2020). Kinetiсs of N-oxyl radicals’ decay. Journal of Organic Chemistry, 85(11), 7112–7124. https://doi.org/10.1021/acs.joc.0c00506
Kushch, O. V., Hordieieva, I. O., Kompanets, M. O., Zosenko, O. O., Opeida, I. A., Shendrik, A. N. (2021). Нydrogen atom transfer from benzyl alcohols to N-oxyl radicals. Reactivity parameters. Journal of Organic Chemistry, 86(5), 3792–3799. https://doi.org/10.1021/acs.joc.0c02595
Zhuk, T., Babkina, V. (2023). [Potential of N-hydroxyphthalimide for large-scale CH-oxidations]. European Science, 3(16-03), 65–83. (in Ukrainian). https://doi.org/10.30890/2709-2313.2023-16-03-014
Koshino, N., Saha, B., Espenson, J. H. (2003). Kinetic Study of the Phthalimide N-Oxyl Radical in Acetic Acid Hydrogen Abstraction from Substituted Toluenes, Benzaldehydes, and Benzyl Alcohols . Journal of Organic Chemistry, 68(24), 9364–9370. https://doi.org/10.1021/jo0348017
Нryshсhenko, Н. O., Nesterova, O. Ju., Kompanets, M. O., Vynokurova, T. K. (2014). [Autooxidation and kinetic aspects of 1.4-dihydropyridines in the presence of the Со(ІІ)/NНРІ system]. Collection of scientific works of the Dniprodzerzhyn State Technical University. Technical sciences, 2. 159–163. (in Ukrainian). http://nbuv.gov.ua/UJRN/Znpddtu_2014_2_32
Нryshсhenko, Н. O. (2017). [Oxidation of 1.4-dihydropyridine derivatives in the presence of (СН3СОО)2Со/phthalimide derivatives]. Internauka: scientific journal, 3(9(13)), 74–75. (in Ukrainian).
Нryshсhenko, Н. O., Nesterova, O. Ju., Kompanets, M. O. (2013). [Aerobic oxidation and kinetic aspects of 1.4-dihydropyridines in the presence of Cо(II) and NHPI]. Bulletin of Dnipropetrovsk University. «Rocket and space technology» series, 2(17), 29–37. (in Ukrainian).
Нryshсhenko, Н. O. (2016). [Reactions of 1-N-substituted pyridine salts with phthalimide-N-oxyl salts]. Modern trends in the development of science in Ukraine: materials of the international scientific and practical conference of young scientists, Rivne, 171. (in Ukrainian).
Yang, Ch., Farmer, L. A., Pratt, D. A., Maldonado, S., Stephenson C. R. J. (2021). Mechanism of Electrochemical Generation and Decomposition of Phthalimide-N-oxyl. Journal of the American Chemical Society, 143(27), 10324–10332. https://doi.org/10.1021/jacs.1c04181
Cinco, M. Á. B., Wu, G., Telser, J., Hayton, T. W. (2022). Structural and Spectroscopic Characterization of a Zinc-Bound N-Oxyphthalimide Radical. Inorganic Chemistry, 61(34), 13250–13255. https://doi.org/10.1021/acs.inorgchem.2c01765
Anderson, T. E., Woerpel, K. A. (2020). Strain-Promoted Oxidation of Methylenecyclopropane Derivatives using N-Hydroxyphthalimide and Molecular Oxygen in the Dark. Organic Letters, 22(14), 5690–5694. https://doi.org/10.1021/acs.orglett.0c02075
[Dekamin, M. G., Moghaddam, F. M., Saeidian, H., Mallakpour, Sh. (2006). The Performance of Phthalimide-N-oxyl Anion. Monatshefte fur Chemie Chemical Monthly, 137, 1591–1595. https://doi.org/10.1007/s00706-006-0553-6
Mauzerall, D., Westheimer, F. H. (1955). 1-Benzyldihydronicotinamide – A Model for Reduced DPN. Journal of the American Chemical Society, 77(8), 2261–2264.
https://doi.org/10.1021/ja01613a070
Nowak, C., Pick, A., Lommes, P., Sieber, V. (2017). Enzymatic Reduction of Nicotinamide Biomimetic Cofactors Using an Engineered Glucose Dehydrogenase: Providing a Regeneration System for Artificial Cofactors. ACS Catalysis, 7(8), 5202–5208. https://doi.org/10.1021/acscatal.7b00721
Lo, H. Ch., Leiva, C., Buriez, O., Kerr, J. B., Olmstead, M. M., Fish, R. H. (2001). Bioorganometallic Chemistry. Regioselective Reduction of NAD+ Models, 1-Benzylnicotinamde Triflate and β-Nicotinamide Ribose-5‘-methyl Phosphate, with in Situ Generated [Cp*Rh(Bpy)H]+: Structure-Activity Relationships, Kinetics, and Mechanistic Aspects in the Formation of the 1.4-NADH Derivatives. Inorganic Chemistry, 40(26), 6705–6716.
https://doi.org/10.1021/ic010562z
Sadri, Z., Behbahani, F. K. (2020). Synthesis of Spiro1.4-Dihydropyridines: A Review. Current Organic Synthesis, 17(5), 324–343. https://doi.org/10.2174/1570179417666200415150027
Soma, M., Yamagishi, A. (1970). Photo-induced electron transfer from organic crystals to biologically interesting substances. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 205, 183–189. https://doi.org/10.1016/0005-2728(70)90248-3
Kazitsyna, L. A., Kupletskaya, N. B. (1979). [Application of UV, IR, NMR and mass spectroscopy in organic chemistry]. Moskow, USSR: Moscow University Press. (in Russian).
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

