COMPLEXATION OF Ge (IV) WITH 6,7-DIHYDROXYBENZOPYRYLLIUM DERIVATIVES AND THEIR ANALYTICAL APPLICATION
DOI:
https://doi.org/10.15421/jchemtech.v31i3.287489Keywords:
6,7-dihydroxybenzopyrylium salts, germanium (IV), complexation, spectrophotometry, optical emission spectroscopy with inductively coupled plasmaAbstract
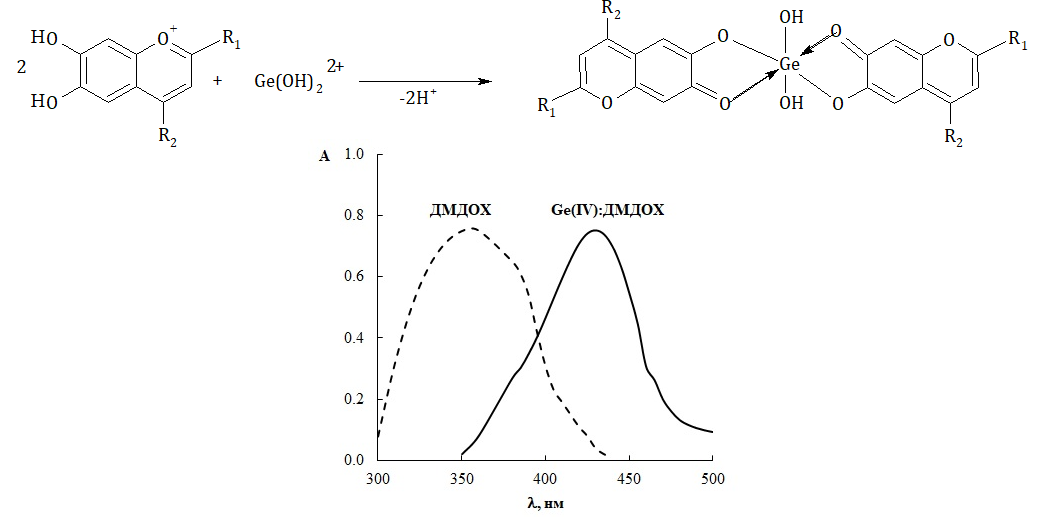
In the current study, the formation of germanium (IV) complexes with some alkyl and phenyl derivatives of 6,7-dihydroxybenzopyrylium was studied. The stoichiometry of Ge(IV) : R = 1 : 2 complexes was established by classical spectrophotometric methods such as the molar ratio method and the equilibrium shift method. On the basis of the data set, the mechanism of complex formation is proposed. It is shown that the complexing agent is the cation Ge(OH)22+, and the ligand interacts in the form of an anhydride base. The chemical-analytical characteristics of the complexes were determined, and it was noted that the most intensely colored and stable are Ge (IV) complexes with 6,7-dihydroxy-2,4-diphenylbenzopyrylium and 6,7-dihydroxy-4-methyl-2-phenylbenzopyrylium salts. It is shown that the introduction of phenyl substituents in positions 2 and 4 of the benzopyrylium fragment leads to a shift of complex formation to a more acidic region, an increase in complex stability, and an increase in their molar absorptivity. Using 6,7-dihydroxy-2,4-dimethylbenzopyrylium salts, a technique for determining germanium (IV) after its extraction and separation in the form of tetrachloride was developed. The method was tested in the analysis of standard reference materials of sludge, coke and dietary supplement samples.
References
Tarafder, P. K., Mondal, R. K. (2011). A review on the complex forming ability of O-O′ type ligands with transition metals: Introducing 2,3-dihydroxynaphthalene as a potential analytical reagent, Rev. Anal. Chem., 30(2), 73–81. https://doi.org/10.1515/REVAC.2011.016
Savvin, S. B., Shtykov, S. N., Mikhailova, A. V. (2006). Organic reagents in spectrophotometric methods of analysis, Russ. Chem. Rev., 75(4), 341–349. https://doi.org/10.1070/RC2006v075n04ABEH001189
Snigur, D., Barbalat, D., Chebotarev, A., Synievyd, A., Bevziuk, K. (2021). A rapid cloud point extraction of Molybdenum(VI) with 6,7-dihydroxy-2,4-diphenylbenzopyrylium perchlorate prior to its spectrophotometric determination. Chem. Papers. 75, 1823–1830. http://dx.doi.org/10.1007/s11696-020-01436-3.
Zhukovetska, O. M., Guzenko, E. M., Chebotarev, A. N., Snigur, D. V. (2022). [Solid-phase spectrophotometric determination of Mo(VI) using organopolymeric cation exchange resin KU-2-8 modified by 6,7-dihydroxy-2-phenyl-4-methylbenzopyrylium chloride]. Methods Objects Chem. Anal. 17, 10–16. (in Ukrainian)
http://dx.doi.org/10.17721/moca.2022.10-16
Snigur, D., Azooz, E. A., Zhukovetska, O., Guzenko, O., Mortada, W. (2023). Recent innovations in cloud point extraction towards a more efficient and environmentally friendly procedure, TrAC, Trends Anal. Chem., 164, 117113. https://doi.org/10.1016/j.trac.2023.117113
Snigur, D., Barbalat, D., Fizer, M., Chebotarev, A., Shishkina, S. (2020). Synthesis and properties of 6,7-dihydroxybenzopyrylium perchlorate halogen derivatives: X-ray, spectroscopic and theoretical studies. Tetrahedron. 76, 131514. https://doi.org/10.1016/j.tet.2020.131514.
Fizer, M., Fizer, O., Barbalat, D., Shishkina, S., Snigur, D. (2022). Structural peculiarities of new benzopyrylium dyes: X-ray, FT-IR, and DFT complex study. J. Molec. Struct. 1252, 132178. https://doi.org/10.1016/j.molstruc.2021.132178.
Sabarudin, A., Umemura, T., Motomizu, S. (2011). Chitosan functionalized with di-2-propanolamine: Its application as solid phase extractant for the determination of germanium in water samples by ICP-MS. Microchem. J. 99, 34–39. https://doi.org/10.1016/j.microc.2011.03.004
Ponomarenko, O., Samchuk, A., Vovk, K., Shvaika, I., Grodzinskaya, G. (2019). Germanium determination in environmental object by the method of mass spectrometry with inductively coupled plasma. Ukrainian Chemistry Journal, 85(4), 110–113. https://doi.org/10.33609/0041-6045.85.4.2019.110-113
McMahon, M., Regan, F., Hughes, H. (2006). The determination of total germanium in real food samples including Chinese herbal remedies using graphite furnace atomic absorption spectroscopy. Food Chem. 97, 411–417. https://doi.org/10.1016/j.foodchem.2005.05.018
Matusiewicz, H., Krawczyk, M. (2000). Determination of germanium and tin and inorganic tin species by hydride generation in situ trapping flame atomic absorption spectrometry. Anal. Lett. 43, 2543–2562. https://doi.org/10.1080/00032711003725631
Boÿkübayram, A. E., Volkan, M. (2000). Cloud point preconcentration of germanium and determination by hydride generation atomic absorption spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 55: 1073–1080. https://doi.org/10.1016/S0584-8547(00)00233-0
Schreiter, N., Wiche, O., Aubel, I., Roode-Gutzmer, Q., Bertau, M. (2021). Determination of germanium in plant and soil samples using high-resolution continuum source graphite furnace atomic absorption spectrometry (HR CS GFAAS) with solid sampling, Journal of Geochemical Exploration, 220, 106674, https://doi.org/10.1016/j.gexplo.2020.106674.
Kaya, M., Volkan, M. (2011). Germanium determination by flame atomic absorption spectrometry: An increased vapor pressure-chloride generation system, Talanta, 84(1), 122–126. https://doi.org/10.1016/j.talanta.2010.12.029
Skwarczynska-Wojsa, A.L., Piech, A. & Wojton, A. (2021). Determination of germanium and other trace elements concentration in mineral waters of Low Beskid (Poland) used for crenotherapy. Environ Earth Sci, 80, 57 https://doi.org/10.1007/s12665-020-09344-1
Ezer, M., Gondi, R., Kennehan, E., Simeonsson, J. B. (2019). Trace determination of Germanium by continuous flow hydride generation laser-induced fluorescence spectrometry, Anal. Lett., 52(7), 1125–1137. https://doi.org/10.1080/00032719.2018.1521827
Gökmeşe, F., Gökmeşe, E., Solak, A.O. (2008) A new adsorptive square-wave stripping voltammetric method for the trace analysis of germanium. Hacettepe J. Biol. Chem. 36, 215–221.
Nazarenko, V. A., Antonovich, V. P. (1973). [Trioxyfluorones]. Moscow, USSR: Nauka (in Russian).
Soylak, M., Yigit, S. (2015) Preconcentration–separation of germanium at ultra-trace levels on polysulfone membrane filter and its determination by spectrophotometry. J. Ind. Eng. Chem. 24, 322–325. https://doi.org/10.1016/j.jiec.2014.10.003
Tomita, H., Samukawa, N., Asano, M., Yamaguchi, T., Matsumura, H., Fujita, Y. (2016) Spectrophotometric determination of germanium(IV) and organogermanes with o-sulfophenylfluorone. Bunseki kagaku. 65, 465–470. https://doi.org/10.2116/bunsekikagaku.65.465
Ivanyca, L.A., Klymkyna, A.Ju., Chmylenko, T.S., Chmylenko F.A. (2016). [Determination of tin and germanium with nonylfluorone and polymer flocculants in plant materials], Vìsnik Dnìpropetrovs’kogo unìversitetu. Serìâ hìmìâ, 24(1), 27–35. (in Ukrainian) https://doi.org/10.15421/081605
Marchenko, Z., Bal’tsezhak, M. (2007). [Methods of spectrophotometry in UV and visual regions in Inorganic Analysis]. Moscow, BINOM. Laboratoriya znanii. 2007. (in Russian).
Selivanova, T., Vishnikin, A., Tsiganok, L. (2020). Visual test determination of trace amounts of germanium in the form of an ionic associate of 12-molybdogermanate with astrafloxin, E3S Web of Conferences, 166, 01013. https://doi.org/10.1051/e3sconf/202016601013
Chyvyreva, N. A., Stojanova, Y. V., Antonovych, V. P., Zynchenko, V. F., Chuhryj, Ju. P. (2018) [Detection and determination of chemical forms of germanium in objects of various natures], Visn. Odes. nac. univ., Him., 23(4), 6–22. http://dx.doi.org/10.18524/2304-0947.2018.4(68).147811
Barbalat, D.A., Chebotarev, A.N., Snigur, D.V. (2020) Anion nature influence on spectral and some physico-chemical properties of 6,7-dihydroxy-4-methyl-2-phenylchromenylium salts. Russ. J. Gen. Chem., 90(4), 597–601. https://doi.org/10.1134/S1070363220040064
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

