PHASE EQUILIBRIA IN THE SYSTEM BASED ON CERIUM DIOXIDE AND LANTHANUM AND YTTERBIUM OXIDES AT A TEMPERATURE OF 1100 оС
DOI:
https://doi.org/10.15421/jchemtech.v32i1.290443Keywords:
phase equilibria, phase diagram, solid solution, lattice parameters, functional ceramicsAbstract
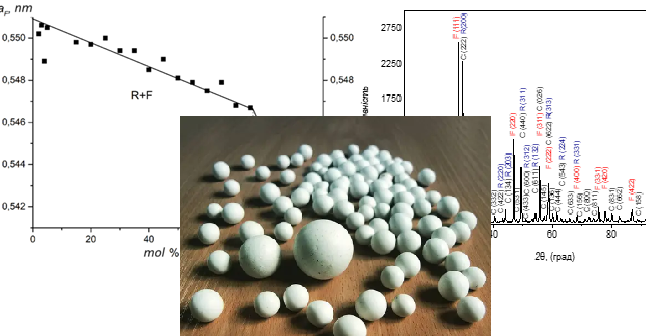
Using information available in the literature, it has been established that in recent times, researchers worldwide have shown increasing interest in materials based on cerium oxide doped with rare earth oxides. As known, phase equilibria in multi-component oxide systems serve as the physicochemical foundation for the development of new materials with improved properties. One of the significant tasks when studying phase equilibria in multi-component systems is to determine the stability boundaries of solid solutions within a specific temperature and concentration range, as well as to identify the existence of ordered phases. In the present study, phase equilibria in the ternary CeO2–La2O3–Yb2O3 system have been investigated over the entire concentration range. An isothermal section of the phase diagram for the CeO2–La2O3–Yb2O3 system at a temperature of 1100 °C has been constructed during the research. The obtained results indicate the absence of new phase formation in the investigated system under the utilized technological conditions. Using X-ray phase analysis, it has been determined that the investigated system exhibits the formation of solid solutions based on the (F) modification of CeO2 with a fluorite-like structure, cubic (C) and hexagonal (A) modifications of rare earth element oxides, as well as an ordered phase (R) crystallizing in a perovskite-like structure with rhombohedral distortion. The solubility of CeO2 in the crystalline lattice of the ordered perovskite-like phase is approximately 2 mol. %. It has been established that this isothermal section is characterized by the formation of two three-phase regions (A+F+R, R+C+F) and five two-phase regions (A+F, A+R, F+R, C+F, C+R). The majority of the mentioned isothermal section is occupied by the three-phase regions.
References
Artini, C. (2018). Rare-Earth-Doped Ceria Systems and Their Performance as Solid Electrolytes: A Puzzling Tangle of Structural Issues at the Average and Local Scale. Inorg. Chem., 57, 13047−13062. https://doi.org/10.1021/acs.inorgchem.8b02131
Sartori da Silva, F., Miguel de Souza, T. (2017). Novel materials for solid oxide fuel cell technologies: A literature review. Inter. J. Hyd. Ener., 42, 26020-26036. doi:10.1016/j.ijhydene.2017.08.105
Liang, S., Wang, H., Li, Y., Qin, H., Luo, Z., Huang, B., Zhao, X., Zhao, C., Chen, L. (2020). Rare-earth based nanomaterials and their composites as electrode materials for high performance supercapacitors: a review. Sustain. Ener. Fuels, 3825–3847. https://doi.org/10.1039/D0SE00669F
Zhang, H., Sun, J., Duo, S., Zhou, X., Yuan, J., Dong, S., Yang, X., Zeng, J., Jiang, J., Deng, L., Cao, X. (2019). Thermal and Mechanical Properties of Ta2O5 Doped La2Ce2O7 Thermal Barrier Coatings Prepared by Atmospheric Plasma Spraying. J. of the Eur. Ceram. Soc, 39, 2379–2388. DOI:10.1016/j.jeurceramsoc.2019.02.041
Zhou, Y., Li, S., Deng, J., Xiong, L., Wang, J., Chen, Y. (2018). Nanoscale heterogeneity and lowtemperature redox property of CeO2-ZrO2-La2O3-Y2O3 quaternary solid solution. Mater.Chem. and Phys., 208, 123–131. https://doi.org/10.1016/j.matchemphys.2018.01.004
Tauseef, M., Faisl, M., Muhammad, S., Muhammad, R. (2020). Novel photocatalyst and antibacterial agent; direct dual Z-scheme ZnO–CeO2-Yb2O3 heterostructured nanocomposite. Sol. Stat. Scien., 109, 106446–106458. https://doi.org/10.1016/j.solidstatesciences.2020.106446
Coduri, M., Checchia, S., Longhi, M., Ceresoli, D., Scavini, M. (2018). Rare Earth Doped Ceria: The Complex Connection Between Structure and Properties. Front. Chem., 6, 526. https://doi.org/10.3389/fchem.2018.00526
Artini, C., Presto, S., Viviani, M., Massardo, S., Carnasciali, M. M., Gigli, L., Pani, M. (2021). The role of defects association in structural and transport properties of the Ce1-x (Nd0.74 Tm0.26)xO2-x/2 system. J. of Ener. Chem., 60, 494–502. doi:10.1016/j.jechem.2020.11.030
Jun-Gill, K., Young-Il, K., Dae Won, C., Youngku, S. (2015). Synthesis and physico chemica lproperties of La(OH)3 and La2O3 nanostructures. Materials Sciencein Semiconductor Processing, 40, 737–743. http://dx.doi.org/10.1016/j.mssp.2015.07.050
Meiser, F., Cortz, C., Caruso, F. (2004). Biofunctionalization of fluorescent rare-earthdoped lanthanum phosphate colloidal nanoparticle. Angew. Chem. Int. Ed., 43, 5954–5957. doi: 10.1002/anie.200460856
Lavrynenko, O.M., Zahornyi, M. M., Vember, V. V. , Pavlenko, O. Yu., Lobunets, T. F., Kolomys, O. F., Povnitsa, O. Yu., Artiukh, L. O., Naumenko, K. S., Zahorodnia, S. D., Garmasheva, I. L. (2022). Nanocomposites Based on Cerium, Lanthanum, and Titanium Oxides Doped with Silver for Biomedical Application. Condens. Matter, 7(3), 45. https://doi.org/10.15407/hftp14.02.262
Zahornyi, M. M., Lavrynenko, O. M., Pavlenko, O. Yu, Povnitsa, O. Yu, Artiukh, L. O., Naumenko, K. S., Zahorodnia, S. D., Ievtushenko, A. I. (2023). The antiviral activity of cerium and lanthanum nanooxides modified with silver. Chemistry, Physics and Technology of Surf., 14, 262–272. doi:10.15407/hftp14.02.262
Soni, S., Kumar, S., Dalela, B., Kumar, Sh., Alvi, P.A., Dalela, S. (2018). Defects and oxygen vacancies tailored structural and optical properties in CeO2 nanoparticles doped with Sm3+ cation. Journal of Alloys and Compounds, 752, 520–531. DOI:10.1016/j.jallcom.2018.04.157
Khakhal, H.R., Kumar, S., Patidar, D., Kumar, Sh., Vats, V.S., Dalela, B., Alvi, P.A., Leel, N.S., Dalela, S. (2023). Correlation of oxygen defects, oxide-ion conductivity and dielectric relaxation to electronic structure and room temperature ferromagnetic properties of Yb3+ doped CeO2 nanoparticles. Materials Science and Engineering: B, 297, 116675. http://dx.doi.org/10.2139/ssrn.4376999
Andrievska, E. R., Kornienko, O. A., Sameliuk, A. V.,Sayir, A. (2011). Phase Relation Studies in the CeO2–La2O3 System at 1100–1500 °С. J. Eur. Ceram. Soc., 31, 1277–1283. https://doi.org/10.1016/j.jeurceramsoc.2010.05.024
Andrievska, E. R., Kornienko, O.A., Sameliuk, A.V., Sair, A. (2020) Phase relation studies in the CeO2-Eu2O3 system at 1500 to 600 °C in air. J. Eur. Ceram. Soc., 40, 751–758. https://doi.org/10.1016/j.jeurceramsoc.2019.10.045
Аndrievskaya, E. R., Kornienko, O. A., Bykov, O. І., Sameliuk, A. V., Bohatyriova, Z. D. (2019). Interaction of ceria and ytterbia in air within temperature range 1500–600°C. J. Eur. Ceram. Soc., 39, 2930–2935. https://doi.og/10.1016/j.jeurceramsoc.2019.03.021
Аndrievskaya, O. R., Коrniienko, O. А., Bykov, O. І., Sameliuk, А. V., Bohatyriova, Z. D. (2020). Interaction of ceria and erbia in air within temperature range 1500–600 °C. J. Eur. Ceram. Soc., 40, 3098–3103. https://doi.org/10.1016/j.jeurceramsoc.2020.03.002
Samoilova, O., Mikhalov, G., Makrvets, L. (2017). Thermodynamic description of phase equilibria in the Cu2O–CeO2–Ce2O3–La2O3 system. Bull. of the South Ural Stat. Univers. Ser. Metall., 17, 16–23. doi:10.14529/met170102
Hrovat, M., Samardžzija, Z., Holc, J., Bernik, S. (1999). Subsolidus phase equilibria in the La2O3–Ga2O3–CeO2 system. J. of Mater. Resear., 14, 4460–4462. https://doi.org/10.1023/A:1006714909627
Małecka, M. A., Kępiński, L. (2010). Structural characterization of nano-sized Ce0.5Ln0.5O1.75 (Ln =Yb, Lu) mixed oxides. J. of Microsc., 237, 391–394. doi: 10.1111/j.1365-2818.2009.03268.x
Mandal, B. P., Grover, V., Roy, M., Tayagi, A. K. (2007). X-Ray diffraction and raman spectroscopic Investigation on the phase relation in Yb2O3- and Tm2O3- substituted CeO2. J. of Amer. Soc., 90, 2961–2965. DOI:10.1111/j.1551-2916.2007.01826.x
Kornienko, O. A., Andrievskaya, O.R., Barshchevskaya, H.K. (2021). Phase relations in the system ternary based on ceria, zirconia and ytterbia at 1500° С. J. of Chem. And Technol., 28, 142 152. https://doi.org/10.15421/082015
Ilatovskaia, M., Sun, S., Saenko, I., Savinykh, G., Fabrichnaya, O. (2020). Experimental Investigation of Phase Relations in the ZrO2-La2O3-Yb2O3 System. J. of Phas. Equilib. And Diff., 41, 311–328. doi:10.1007/s11669-020-00790-9
Coutures, J., Rouanet, A., Verges, R., Foex, M. (1976). Etude a haute temperature des systems formes par le sesquioxyde de lanthane et les sesquioxydes de lanthanides. I: Diagrammes de phases (1400 ℃ < T < T Liquide). J. Solid State Chem., 17, 172–182. https://doi.org/10.1016/0022-4596(76)90218-8
Muller–Buschbaum Hk., Teske Chr. L. (1969). Zur Kenntnis der Kristallstruktur von LaYbO3. Z. Anorg. Allg. Chem., 369, 255–264. https://doi.org/10.1002/zaac.19693690316
Muller–Buschbaum Hk. (1969). Untersuchung am System La2O3–Yb2O3. Z. Anorg. Allg. Chem. Bd., 369, 249–254. https://doi.org/10.1002/zaac.19693690315
Traverse J. P., Coutures J., Foex M. (1968).Thermal analysis. Compt. Rend. Acad. Sci., 924–935.
Chudinivych O. V., Andrievskaya O.R., Bohatyriova Z. D. (2014). Interaction of lanthanum and ytterbia at 1600 °C 1500 °С. Curr. Prob. of Phys. Mater. Sci., 23, 12–23.
Chudinovych, О. V., Bykov, O. I., Samelyuk, A. V. (2021). Phase relation studies in the La2O3–Lu2O3–Yb2O3 system at 1500 °С. J. Chem. and Techn., 29(4), 485–494. https://doi.org/10.15421/jchemtech.v29i4.238943
Chudinovych, O., Bykov, O., Samelyuk, A. (2021). Interaction of Lanthanum, Lutetium, and Ytterbium Oxides at 1600°C. Powder Metall Met Ceram 60, 337–345 https://doi.org/10.1007/s11106-021-00248-8
Soloviova, A. E. (2010). Simulation of the formation and dissociation processes of solid solution in СеО2- La2O3 system. Journal of nano- and electronic physics, 1, 30-37.
Lavrynenko, O. M., Bykov, O. I., Bataiev, Y. M., Bataiev, M. M., Kornienko, O. A. (2020). Influence of temperature on the formation of structures in the CeO2-Yb2O3 system. Vìsnik Odesʹkogo Nacìonalʹnogo Unìversitetu: Hìmìâ, 25, 3(75), 76–85. https://doi.org/10.18524/2304-0947.2020.3(75).208388
Ito, K., Tezuka, K., Hinatsu, Y. (2001). Preparetion, Magnetic Susceptibility, and Specific Heat on Interlathanide Perovskites ABO3 (A =La-Nd, B = Dy – Lu). J. Solid State Chem., 157, 173–179. DOI:10.1006/jssc.2000.9071
Obukuro, Yu., Ninomiya, K., Arai, M., Okuyama, Yu., Sakai, G., Matsushima, Sh. (2017) First-principles study on LaYbO3 as the localized f electrons containing system with MBJ–LDA + U approach. Computational Materials Science, 126, 7–11. doi:10.1016/j.commatsci.2016.09.005
Kasyanova, A. V., Lyagaeva, J. G., Vdovin, G. K., Murashkina, A. A., Medvedev, D. A. (2023). Transport properties of LaYbO3-based electrolytes doped with alkaline earth elements. Electrochimica Acta, 439, 141702. https://doi.org/10.1016/j.electacta.2022.141702
Obukuro, Y., Ninomiya, K., Arai, M., Okuyama, Y., Sakai, G., Matsushima, S. (2017). First-principles study on LaYbO3 as the localized f electrons containing system with MBJ–LDA + U approach. Computational Materials Science, 126, 7–11. https://doi.org/10.1016/j.commatsci.2016.09.005
Su W., Yang L., Li B. (2011). Optical properties and thermal stability of LaYbO3 ternary oxide for high-k dielectric application. Applied Surface Science, 257, 7, 2526–2530. https://doi.org/10.1016/j.apsusc.2010.10.016
Kornienko O., Yushkevych S., Bykov O., Samelyuk A., Bataiev Y. (2022). Phase Equilibrium in the Ternary CeО2–La2O3–Yb2O3 System at 1500 °С. Solid State Phenomena, 331, 159-172 https://doi.org/10.4028/p-4000g3
Kornienko O.A., Sameljuk A.V., Bykov О. І., Yurchenko Yu.V., Barshchevskaya A. K. (2020) Phase Relation Studies in the CeO2–La2O3–Er2O3 System at 1500°C. Journal of the European Ceramic Society, 40, P. 4184–4190. https://doi.org/10.1016/j.jeurceramsoc.2020.04.042
Xiang P., Ismail S. A., Guo Sh., Jiang L., Han D. (2024) Fluorite-based proton conducting oxides: structures, materials and applications. Materials Advances, 5, 12–29. doi: 10.1039/D3MA00367A
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

