PYRIDINE SALTS WITH MONO- AND DI-N-OXYPHTHALIMIDE ANION
DOI:
https://doi.org/10.15421/jchemtech.v31i4.292325Keywords:
pyridine salts; cordiamine; niketamide; nicotinic acid diethylamide; N-hydroxyphthalimide; N-oxyphthalimide anion.Abstract
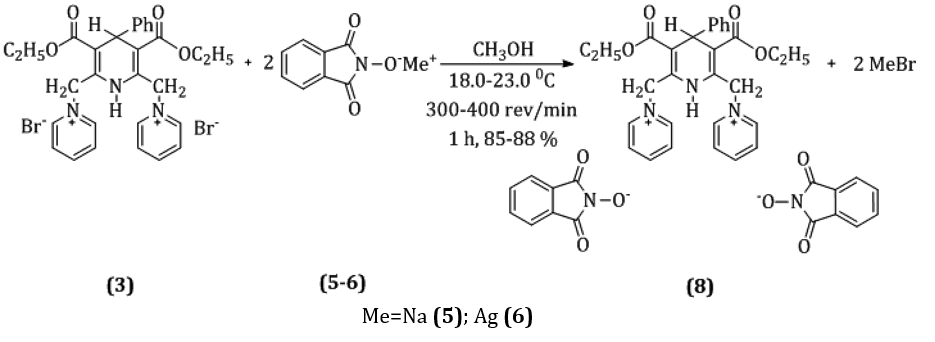
Chemical modification of heterocyclic derivatives of pyridine salts of nicotinamide is a rather promising method of manufacturing medicinal products, considering their wide range of biological effects. Pyridine salts are also used in the production of aromatic substances, in organic synthesis, as well as in the synthesis of polymer products. Some of their derivatives are biologically and physiologically active compounds. However, modern trends in "green" chemistry involve the use of inexpensive reagents under the most mild conditions to obtain target products almost quantitatively. Therefore, we synthesized and studied new water-soluble derivatives of pyridine salts with mono- and di-N-oxyphthalimide anion. The reactions were carried out by the interaction of a pyridine molecule (mono- and bicationic structure) with phthalimide-N-oxyl sodium or argentum salt. These salts were obtained from N-hydroxyphthalimide, which is an effective catalyst for radical reactions of 1.4-dihydropyridine oxidation with molecular oxygen followed by the formation of a bond with the phthalimide-N-oxyl anion in the synthesis of pyridine salts. The method developed by us for the synthesis of pyridine salt derivatives with phthalimide-N-oxyl anion was based on the mono- and bicationic structures of these heterocyclic compounds.
References
Нryshсhenko, Н. O., Didenko, O. S., Kompanets, M. O., Smoljnykova, T. Ju. (2014). [Pyridinium salts with N-oxyphthalimide anion]. ХІІ All-Ukrainian conference of young scientists and students on topical issues of chemistry, Dnipropetrovsk, 91 (in Ukrainian).
Нryshсhenko, Н. O., Didenko, O. S., Smoljnykova, T. Ju., Nesterova, Ye. Yu. (2016). [Derivatives of pyridine salts of dilactone with phthalimide-N-oxyl anion]. XII All-Ukrainian conference of young scientists and students on topical issues of chemistry. Collection of works, Kharkiv, 8 (in Ukrainian).
Nesterova, Ye. Yu., Нryshсhenko, Н. O. (2013). [The main directions of development of the chemistry of 1.4-dihydropyridines – a review of the literature]. Bulletin of Dnepropetrovsk University. Series «Chemistry», 21(19), 61–79 (in Russian). https://doi.org/10.15421/081308
Mesropyan, E. G., Buniatyan, Yu. A., Karapetyan, Z. T., Dang, M. T. (1969). [Method for producing spiro-y, y-dilactone-bis-(1.2-dioxypropyl-2-hydroxyethylmalonic ester) or spiro-y, y-dilactone-bis-(di-(1.2-dioxypropyl))malonic ester]. Pat. SU 271510 USSR, IPC C 07d 5/06. (No. 1301355/23-4) with the addition of application (No 1301356) 23-4. (in Russian).
Akhnazaryan, A. A., Khachatryan, L. A., Dangyan, M. T. (1969). [Method for preparing substituted y,y-dilactones of dicarboxylic acids] Pat. SU 317652 USSR, IPC C 07d 5/06. Declared on 18.III.1969 (No 1312896 23-4) with the addition of application No.; publ. 19.X. (in Russian).
Plotniece, A., Pajuste, K., Kaldre, D., Cekavicus, B., Vigante, B., Turovska, B., Belyakov, S., Sobolev, A., Duburs, G. (2009). Oxidation of cationic 1.4-dihydropyridine derivatives as model compounds for putative gene delivery agents. Tetrahedron, 65(40), 8344–8349. doi:10.1016/j.tet.2009.08.012
Rucins, M., Kaukulis, M., Plotniece, A., Pajuste, K., Pikun, N., Sobolev, A. (2022). 1.1′-{[3.5-Bis(dodecyloxycarbonyl)-4-(naphthalen-2-yl)-1.4-dihydropyridine-2.6-diyl]bis(methylene)}bis{4-[(E)-2-(naphthalen-2-yl)vinyl]pyridin-1-ium}dibromide. Molbank, 2022(3), M1396. https://doi.org/10.3390/M1396
Rucins, M., Pajuste, K., Sobolev, A., Plotniece, M., Pikun, N., Pajuste, K., Plotniece, A. (2020). Data for the synthesis and characterisation of 2.6-di(bromomethyl)-3.5-bis(alkoxycarbonyl)-4-aryl-1.4-dihydropyridines as important intermediates for synthesis of amphiphilic 1.4-dihydropyridines. Data in Brief, 30, 105532. https://doi.org/10.1016/j.dib.2020.105532
Serhieieva, Н. O., Kompanets, M. O., Nesterova, O. Ju. (2023). [N-substituted pyridine salts with phthalimide-N-oxyl anion]. Journal of Chemistry and Technologies, 31(3), 437–442 (in Ukrainian). doi: 10.15421/jchemtech.v31i3.286682
Chen, Ma. R., Wang, W., Yi, L., Xiao, X., Gao, Y., Zhang, X., Tang, J., Yang, X., Meng, Ch., Zheng, X., Xiao, A. (2019). N-oxyl radicals trapped on zeolite surface accelerate photocatalysis. ACS Catalysis, 9(11), 10448–10453. https://doi.org/10.1021/acscatal.9b03737
Mancini, M. Di B., Gelsomino, A. D., Stefano, S. Di, Frateloreto, F., Lapi, A., Lanzalunga, O., Olivo, G., Sajeva, S. (2021). Change of selectivity in C-H functionalization promoted by nonheme iron(IV)-oxo complexes by the effect of the N-hydroxyphthalimide HAT mediator. ACS Omega, 6(40), 26428–26438. https://doi.org/10.1021/acsomega.1c03679
Zhu, Zh., Zhang, Q., Xie, D., Liu, H., Wang, H., Shi, L., Chen, Ch. (2022). Photo- and solvent-mediated production of the highly reactive N-oxyl radical and its efficient catalytic oxidation of hydrocarbons at ambient temperature. ACS Sustainable Chemistry. Engineering, 10(41), 13765–13774. https://doi.org/10.1021/acssuschemeng.2c03985
Kushch, O. V., Hordieieva, I. O., Novikova, K. V., Litvinov, Y. E., Kompanets, M. O., Shendrik, A. N., Opeida, I. A. (2020). Kinetiсs of N-oxyl radicals’ decay. Journal of Organic Chemistry, 85(11), 7112–7124. https://doi.org/10.1021/acs.joc.0c00506
Kushch, O. V., Hordieieva, I. O., Kompanets, M. O., Zosenko, O. O., Opeida, I. A., Shendrik, A. N. (2021). Нydrogen atom transfer from benzyl alcohols to N-oxyl radicals. Reactivity parameters. Journal of Organic Chemistry, 86(5), 3792–3799. https://doi.org/10.1021/acs.joc.0c02595
Zhuk, T., Babkina, V. (2023). [Potential of N-hydroxyphthalimide for large-scale CH-oxidations]. European Science, 3(16-03), 65–83 (in Ukrainian). https://doi.org/10.30890/2709-2313.2023-16-03-014
Yang, Ch., Farmer, L. A., Pratt, D. A., Maldonado, S., Stephenson C. R. J. (2021). Mechanism of electrochemical generation and decomposition of phthalimide-N-oxyl. Journal of the American Chemical Society, 143(27), 10324–10332. https://doi.org/10.1021/jacs.1c04181
Cinco, M. Á. B., Wu, G., Telser, J., Hayton, T. W. (2022). Structural and spectroscopic characterization of a zinc-bound N-oxyphthalimide Radical. Inorganic Chemistry, 61(34), 13250–13255. https://doi.org/10.1021/acs.inorgchem.2c01765
Anderson, T. E., Woerpel, K. A. (2020). Strain-promoted oxidation of methylenecyclopropane derivatives using N-hydroxyphthalimide and molecular oxygen in the dark. Organic Letters, 22(14), 5690–5694. https://doi.org/10.1021/acs.orglett.0c02075
Dubur, G. Ya., Bisenieks, E. A., Uldrikis, Ya. R., Ivanov, Ye. V., Ponomareva, T. V., Merkushev, G. N., Yakubovskiy-Lipskiy, Yu. O. (1990). [Radioprotector "Dieton"]. Latvian SSR patent SU 15636996 (А1). (in Russian).
Нryshсhenko, Н. O. (2016). [Reactions of 1-N-substituted pyridine salts with phthalimide-N-oxyl salts]. Modern trends in the development of science in Ukraine: materials of the international scientific and practical conference of young scientists, Rivne, 171 (in Ukrainian).
Mauricio, J. D. (1963). Nikethamide as a respiratory analeptic. JAMA: The Journal of the American Medical Association, 185(2), 69–74. doi:10.1001/jama.1963.03060020029016.
Eckenhoff, J. E., Hafkenschiel, J. H. (1947). The effect of nikethamide on coronary blood flow and cardiac oxygen metabolism. Journal of Pharmacology and Experimental Therapeutics, 91(4), 362–369.
Murray, W. D., Grant, I. W. B. (1966). Nikethamide and aminophylline in ventilatory failure. Scottish Medical Journal, 11(3), 89–98. doi:10.1177/003693306601100303
Peppin, J. F., Pergolizzi Jr, J. V., Fudin, J., Meyer, T. A., Raffa, R. B. (2021). History of respiratory stimulants. Journal of Pain Research, 14, 1043–1049. doi:10.2147/JPR.S298607
Mardashko, O. O., Jasynenko, N. Je. (2008). [Biological and bioorganic chemistry: Education. manual]. Odesa: Odessa State Medical University. (in Ukrainian).
Нryshсhenko, Н. O., Didenko, O. S., Smoljnykova, T. Ju., Nesterova, Ye. Yu., Kompanets, M. O. (2016). [Pyridinium salts with N-oxyphthalimide anion]. XVІІІ International youth scientific and practical conference "Man and Space", Dnipropetrovsk, 150 (in Ukrainian).
Yedase, G. S., Venugopal, S., Arya, P., Yatham, V. R. (2022). Catalyst-free Hantzsch ester-mediated organic transformations driven by visible light. Asian Journal of Organic Chemistry, 11(10). https://doi.org/10.1002/ajoc.202200478
Sadri, Z., Behbahani, F. K. (2020). Synthesis of spiro1.4-Dihydropyridines: A Review. Current Organic Synthesis, 17(5), 324–343. https://doi.org/10.2174/1570179417666200415150027
Нryshсhenko, Н. O., Kramarjov, S. M. (2017). [Study of the growth-regulating activity of new derivatives of pyridine salts with the anion of N-oxyphthalimide]. Tenth Ukrainian scientific conference of students, postgraduates and young scientists with international participation "Chemical problems of today", Vinnitsa, 120 (in Ukrainian).
Zhuk, T., Babkina, V. (2023). [Potential of N-hydroxyphthalimide for large-scale CH-oxidations]. European Science, 3(16-03), 65–83 (in Ukrainian). https://doi.org/10.30890/2709-2313.2023-16-03-014
Balackaja, T. L., Lavrychenko, Y. V., Нryshсhenko, Н. O. (2013). [Pyridinium salts with N-oxyphthalimide anion]. XI All-Ukrainian conf. of young scientists and students on topical issues of chemistry, Dnipropetrovsk, 96 (in Ukrainian).
Dekamin, M. G., Moghaddam, F. M., Saeidian, H., Mallakpour, Sh. (2006). The performance of phthalimide-N-oxyl anion. Monatshefte fur Chemie Chemical Monthly, 137, 1591–1595. https://doi.org/10.1007/s00706-006-0553-6
Howard, W., Chambers, Casida, J. E. (1967). Chambers protective activity of nicotinic acid derivatives and their 1-alkyl-p- and 1-alkyld-pyridones against selected neurotoxic agents. Toxicology and applied pharmacology, 105–118.
Ivanovskiy, A. P., Shikhanov, V. A., Kutin, A. M., Korshunov, M. A., Kazankin, V. A., Shkarnikov, Ye. N. (1977). [Method for producing alkylpyridines]. Pat. 567724 USSR, IPC C 07D 213/08, No 2047432/04. (in Russian).
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

