ACID-BASE AND ELECTROCHEMICAL BEHAVIOR OF MONOETHANOLAMINE (POLYETHYLENEPOLYAMINE) – CITRIC ACID – WATER SOLUTIONS
DOI:
https://doi.org/10.15421/jchemtech.v32i1.292412Keywords:
ammonium-citrate buffers, cation-molecular complexes, ionic associates, electrical conductivity, densityAbstract
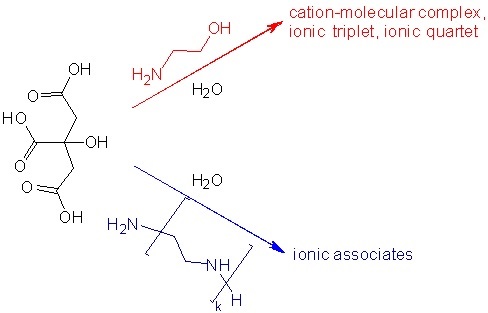
Acid-base and electrochemical behavior in the systems H3Cit – Am – H2O (H3Cit – citric acid; Am – monoethanolamine and polyethylene polyamine) and their structural characteristics features establishment is an urgent task. The pH and conductometric study of protolytic equilibria in the systems HOC3H4(COOH)3 – NH2CH2CH2OH – H2O and HOC3H4(COOH)3 – NH2(CH2CH2NH)kH – H2O was carried out with the total content of citrate forms (citric acid, dihydrogen citrate, hydrogen citrate and citrate anions, cation-molecular complexes and ionic associates) 1.0 M in the temperature range 293 – 313 K. Intermolecular interactions in these systems were estimated in comparison with sodium citrate – citric acid – water solutions at 298 K. The order of components adding affects resulting solutions pH values in contrast to the specific electrical conductivity and density. With the same total citrates content according to the specific electrical conductivity values the studied systems can be arranged in such series: HOC3H4(COONa)3 – HOC3H4(COOH)3 – H2O > NH2CH2CH2OH – HOC3H4(COOH)3 – H2O > NH2(CH2CH2NH)kH – HOC3H4(COOH)3 – H2O. This is because of the mobility of cations and the additional formation of cation-molecular complexes and ionic associates due to H-bonding in the monoethanolamine and polyethylene polyamine systems. According to densitometry, the organic amine (monoethanolamine or polyethylene polyamine) introduction into an citric acid aqueous solution in contrast to sodium citrate results the structuring of the system. The breaks positions on the densitometric curves correspond to the breaks on the conductometric curves; the studied solutions specific electrical conductivity values correlate with their density. The investigated solutions cation-molecular compositions and ionic strengths were calculated. The cation-molecular complexes and ionic associates concentration and thermodynamic formation constants estimation was carried out. The obtained results can be useful to evaluate of the solutions studied in this work buffer properties as well as for developing of chemisorbents based on them.
References
Apelblat, A., Korin, E., Manzurola, E. (2013). Thermodynamic properties of aqueous solutions with citrate ions. Compressibility studies in aqueous solutions of citric acid. J. Chem. Thermodyn. 64, 14–21. http://dx.doi.org/10.1016/j.jct.2013.04.017
Rodnikova, M.N., Agayan, G.M., Balabaev, N.K. (2019). Description of the spatial networks of hydrogen bonds in liquids by topological methods. J. Mol. Liq. 283. 374–379. https://doi.org/10.1016/j.molliq.2019.03.090
Kutus, B., Dudás, C., Friesen, S., Peintler, G., Pálinkó, I., Sipos, P., Buchner, R. (2020). Equilibria and Dynamics of Sodium Citrate Aqueous Solutions: The Hydration of Citrate and Formation of the Na3Cit0 Ion Aggregate. J. Phys. Chem. B. 124(43). 9604–9614. https://doi.org/10.1021/acs.jpcb.0c06377
Lounev, I.V., Rodnikova, M.N., Razumova, A.B., Melnikova T.A. (2023). Mobility of aliphatic amino-alcohols molecules in aqueous solutions investigated by broadband dielectric spectroscopy. J. Mol. Liq. 387, 122674. https://doi.org/10.1016/j.molliq.2023.122674
Legkov, S.A., Bondarenko, G.N., Kostina, J.V., Novitsky, E.G., Bazhenov, S.D., Volkov, A.V., Volkov, V.V. (2023). Structural Features of Monoethanolamine Aqueous Solutions with Various Compositions: A Combined Experimental and Theoretical Study Using Vibrational Spectroscopy. Mol. 28(1), 403. https://doi.org/10.3390/molecules28010403
Patyar, P., Kaur, G. (2022). Molecular interactions of glycine and L-alanine + citrate buffer solutions at different temperatures: Volumetric, viscometric, and FTIR approach. Indian J. Chem. 61(5), 482–495. https://doi.org/10.56042/ijc.v61i5.63636
Apelblat, A. (2014). Citric acid. Springer Cham. https://doi.org/10.1007/978-3-319-11233-6
Del Pino, I.G., Constanso, I., Mourín, L.V., Safont, C.B., Vázquez P.R. (2013). Citric/citrate buffer: an effective antiglycolytic agent. Clinical Chem. Lab. Med. 51(10), 194–204. https://doi.org/10.1515/cclm-2012-0735
Sun, Z., Zhou, Y., Jia, S., Wang, Y., Jiang, D., Zhang, L. (2021). Enhanced SO2 Absorption Capacity of Sodium Citrate Using Sodium Humate. Catalysts. 11(7), 865. https://doi.org/10.3390/catal11070865
Ennan, A.A.-A., Khoma, R.E., Dlubovskii, R.M., Zakharenko, Yu.S., Bienkovska, T.S., Knysh, I.M. (2022). [Mono- and bifunctional impregnated fiber chemosorbents for respiratory purpose]. Visn. Odes. nac. univ., Him. 27(1), 5–30. (in Ukrainian). https://doi.org/10.18524/2304-0947.2022.1(81).248297
Bienkovska, T., Khoma, R., Vatral, O., Dlubovskii, R.M., Vodzinskii, S.V., Menchuk, V.V. (2022). Impregnated fibrous chemisorbents for the colorimetric detection of ammonia. Ukr. Chem. J., 88(12), 175–188. https://doi.org/10.33609/2708-129X.88.12.2022.175-188
Wang, J., Huang, W., Xu, H., Cui, P., Qu, Z., Yan, N. (2023). High-efficient cyclic absorption of sulfur dioxide in Na-Mg-Ci3- compound system for wet flue gas desulfurization. Sep. Purif. Technol. 320, 124138
Fiume, M.M., Heldreth, B.A., Bergfeld, W.F., Belsito, D.V., Hill, R.A., Klaassen, C.D., Liebler, D.C., Marks, J.G., Shank, R.C., Slaga, T.J., Snyder, P.W., Andersen, F.A. (2015). Safety Assessment of Ethanolamine and Ethanolamine Salts as Used in Cosmetics. Int. J. Toxicol. 34(2), 84S–98S. https://doi.org/10.1177/1091581815596439
Baudot, A., Cacela, C., Daurte, M.L., Fausto, R. (2002). Thermal study of simple amino-alcohol solutions. Cryobiol. 44(2), 150–160. https://doi.org/10.1016/s0011-2240(02)00017-2
Yasui, K., Uegaki, M., Shiraki, K., Ishimizu, T. (2010). Enhanced solubilization of membrane proteins by alkylamines and polyamines. Protein Sci. 19, 486–493. https://doi.org/10.1002/pro.326
Khoma, R.E., Ennan, A.A., Dlubovskii, R.M., Ishkov, Yu.V., Bienkovska, T.S., Rakhlitskaya, E.M. (2021). Equilibrium Processes in AlkNHCH2SO3H – NH2CH2CH2OH – H2O Solutions. Russ. J. Gen. Chem. 91(4), 583–592. https://doi.org/10.1134/s1070363221040010
Orzechowski, K., Pajdowska, M., Przybylski, J., Gliński, J., Kołodziej, H.A. (2000). Dielectric, acoustic, densimetric and viscosimetric investigations of the tributylamine + propionic acid system. Phys. Chem. Chem. Phys. 2(20), 4676–4681. https://doi.org/10.1039/B005434H
Burakowski, A., Gliński, J. (2014). Ultrasonic and Densimetric Titration Applied for Acid-base Reactions. Anal. Sci. 30(8), 793–798. https://doi.org/10.2116/analsci.30.793
Ziemer, S.P., Niederhauser, T.L., Merkley, E.D., Price, J.L., Sorenson, E.C., McRae, B.R., Patterson, B.A., Origlia-Luster, M.L., Woolley E.M. (2006). Thermodynamics of proton dissociations from aqueous glycine at temperatures from 278.15 to 393.15 K, molalities from 0 to 1.0 molkg−1, and at the pressure 0.35 MPa: Apparent molar heat capacities and apparent molar volumes of glycine, glycinium chloride, and sodium glycinate. J. Chem. Thermodyn. 38(4), 467–483. https://doi.org/10.1016/j.jct.2005.06.017
Khoma, R.E., Ennan, A.A.-A., Dlubovskii, R.M., Bienkovskaya, T.S. (2021). [Buffer solutions based on taurine]. Visn. Odes. nac. univ., Him. 26(1), 48-64. (in Ukrainian). https://doi.org/10.18524/2304–0947.2021.1(77).226146
de Cássia da Silva, R., Cavalheiro, É.T.G. (2015). Synthesis, characterization, and thermal analysis of alginate and monoethanolamine product. J. Therm. Anal. Calorim. 120(1), 855–862. https://doi.org/10.1007/s10973-014-3948-3
Bone, D.P., Shannon, E.L. (1991). Effects of Order of Mixing and Solute Interactions on the “Water Activity” of Concentrated Solutions. In: Levine H., Slade L. (eds) Water Relationships in Foods. Advances in Experimental Medicine and Biology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-0664-9_18
Khoma, R.E., Bіenkovska, T.S., Osadchiy, L.T., Ishkov, Yu.V. (2023). [Citric acid – sodium citrate – water solutions acid-base and electrochemical behavior]. Visn. Odes. nac. univ., Him. 28(2), 33–42. (in Ukrainian). https://doi.org/10.18524/2304-0947.2023.2(85).286600
Khoma, R.E. (2019). Acid-base interaction and sulfooxidation at chemosorption of sulfur dioxide by alkylamines aqueous solutions. Abstract of Doctor’s degree dissertation. Kyiv. (in Ukrainian)
Zhang H., Lu C.-T., Lu J.-G., Gao L., Hai G.-P., Tang Y.-Q., Chen L.-X., Hua A.-C., Wang L.-J. (2014). Density, Viscosity and Excess Molar Volume of the Aqueous Ionic Liquid Tris(monoethanolamine) Citrate at 293.15–323.15 K. J. Solution Chem. 43, 2117–2132. https://doi.org/10.1007/s10953-014-0270-4
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

