N-BENZYLOXY-N-METHOXYREA. SYNTHESIS AND STRUCTURE
DOI:
https://doi.org/10.15421/jchemtech.v32i1.292868Keywords:
N-acyloxy-N-alkoxyureas; N-alkoxy-N’-сhloroureas; N,N-dialkoxyureas; N-benzyloxy-N’-methoxyurea; synthesis; structure.Abstract
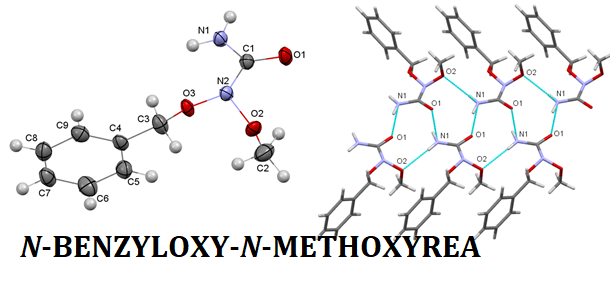
Aim. To synthesize of N-benzyloxy-N-methoxyurea from the methanolysis of N-acetoxy-N-benzyloxyurea and to investigate its structure using the XRD study. Methods. Mass spectrometry, 1H and 13C NMR spectroscopy, IR spectroscopy, XRD study. Results. N-Benzyloxy-N-chlorourea was synthesized with high yield of the N-benzyloxyurea by the chlorination by tert-butyl hypochlorite. We have proved that the interaction of with sodium acetate in acetonitrile medium at room temperature leads to the formation of N-acetoxy-N-benzyloxyurea with the moderate yield. It has been also found that the methanolysis of N-acetoxy-N-benzyloxyurea at room temperature is a convenient method of the N-benzyloxy-N-methoxyurea synthesis. The structure of the synthesized N-benzyloxy-N-chlorourea, N-acetoxy-N-benzyloxyurea and N-benzyloxy-N-methoxyurea was confirmed by the data of 1H and 13C NMR spectra, IR spectra and mass spectra. Also, the XRD study of N-benzyloxy-N-methoxyurea structure proved our assumptions: O–N–O nitrogen atom (N2) in N-benzyloxy-N-methoxyurea is sp3 hybridized and has pyramidal configuration. The carbamoyl nitrogen atom (N1) has planar configuration. After comparison of the amide bond of the received N-benzyloxy-N-metoxyurea with the similar bond in unsubstituted N-benzyloxyurea and H2N(1)–C(=O) bond of N-benzyloxy-N-methoxyurea we have found the significant elongation of the amide BnO(MeO)N(2)–C(=O) bond of N-benzyloxy-N-methoxyurea. The molecular packings in the crystal of N-benzyloxy-N-methoxyurea and N-benzyloxyurea is different. Conclusions. As the result of our study the anomeric N-benzyloxy-N-chlorourea, N-acetoxy-N-benzyloxyurea and N-benzyloxy-N-methoxyurea have been synthesized. The structure of N-benzyloxy-N-methoxyurea has been thoroughly analyzed.
References
Glover, S.A. (1998). Anomeric Amides – Structure, Properties and Reactivity. Tetrahedron, 54(26), 7229–7271.https://doi.org/10.1016/S0040-4020(98)00197-5
Glover, S. A. (2009). N-Heteroatom-substituted hydroxamic esters, in The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids, Eds Rappoport, Z., Liebman, J. F., John. Wiley and Sons, New York. PATAI’S Chemistry of Funcional Groups, 1, 839–923. https://doi.org/10.1002/9780470682531.pat0470
Glover, S.A., Rosser, A.A. (2018). Heteroatom Substitution at Amide Nitrogen – Resonance Reduction and HERON Reactions of Anomeric Amides. Molecules, 23(11), 2384. https://doi.org/10.3390/molecules23112834
Campbell, J. J., Glover, S. A., Hammond, G. P., Rowbottom, C.A. (1991). Evidence for the Formation of Nitrenium Ions in the Acid-catalysed Solvolysis of Mutagenic N-Acetoxy-N-Alkoxybenzamides. J. Chem. Soc., Perkin Trans., 2(12), 2067–2079. https://doi.org/10.1039/P29910002067
Campbell, J.J., Glover, S.A. (1999). Bimolecular Reactions of Mutagenic N-Acyloxy-N-alkoxybenzamides with Aromatic Amines. J. Chem. Reseach (S), (8), 474–475. https://doi.org/10.1039/A903555I
Glover, S.A., Mo, G., Rauk, A., Tucker, D.J., Turner, P. (1999). Structure, conformation, anomeric effects and rotational battiers in the HERON amides, N,N’-diacyl-N,N’-dialkoxyhydrazines. J. Chem. Soc., Perkin Trans., 2, (10), 2053–2058. https://doi.org/10.1039/A904575I
Glover, S.A., Rauk, A. (1999). Conformational Stereochemistry of the HERON Amide, N-Methoxy-N-dimethylaminoformamide: A Theoretical Study. J. Org. Chem., 64(7), 2340–2345. https://doi.org/10.1021/jo982048p
Gillson, A.-M., Glover, S.A., Tucker, D.J., Turner, P. (2003). Crystal structures and properties of mutagenic N-acyloxy-N-alkoxyamides – “most pyramidal” acyclic amides. Org. Biomol. Chem., 1(19), 3430−3437. https://doi.org/10.1039/B306098P
Glover, S.A., White, J.M., Rosser, A.A., Digianantonio, K.M. (2011). Structure of N,N-Dialkoxyamides: Pyramidal Anomeric Amides with Low Amidicity, J. Org. Chem., 76(23), 9757−9763. https://doi.org/10.1021/jo201856u
Digianantonio, K.M., Glover, S.A., Johns, J.P., Rosser, A.A. (2011). Synthesis and thermal decomposition of N,N-dialkoxyamides. Org. Biomol. Chem., 9(11), 4116−4126. https://doi.org/10.1039/C1OB00008J
Shtamburg, V.G., Tsygankov, A.V., Shishkin, O.V., Zubatyuk, R.I., Uspensky, B.V., Shtamburg, V.V., Mazepa, A.V., Kostyanovsky, R.G. (2012). The properties and structure of N-chloro-N-methoxy-4-nitrobenzamide. Mendeleev Commun., 22(3), 164–166. https://doi.org/10.1016/j.mencom.2012.05.019
Cavanagh, K.L., Glover, S.A., Price, H.L., Schumacher, R.R. (2009). SN2 Substitution reactions at the Amide Nitrogen in the Anomeric Mutagens, N-Acyloxy-N-alkoxyamides. Aust. J. Chem., 62(7), 700–710. https://doi.org/10.1071/CH09166
Glover, S.A., Taherpour, A., Greatrex, B.W. (2014). Formation and HERON Reactivity of Cyclic N,N-Dialkoxyamides. Aust. J. Chem., 67(3), 507–520. https://doi.org/10.1071/CH13557
Shishkin, O.V., Zubatyuk, R.I., Shtamburg, V.G., Tsygankov, A.V., Klots, E.A., Mazepa, A.V., Kostyanovsky, R.G. (2006). Pyramidal Amide Nitrogen in N-Acyloxy-N-alkoxyureas and N-Acyloxy-N-alkoxycarbamates, Mendeleev Commun., 16(4), 222−223. https://doi.org/10.1070/MC2006v016n04ABEH002195
Shtamburg, V.G., Shishkin, O.V., Zubatyuk, R.I., Kravchenko, S.V., Tsygankov, A.V., Mazepa, A.V., Klots, E.A., Kostyanovsky, R.G. (2006). N-Chloro-N-alkoxyureas: synthesis, structure and properties. Mendeleev Commun., 16(6), 323–325. https://doi.org/10.1070/MC2006v016n06ABEH002382
Shtamburg, V.G., Shishkin O.V., Zubatyuk R.I., Kravchenko, S.V., Shtamburg, V.V., Distanov, V.B., Tsуgankov A.V., Kostyanovsky, R.G. (2007). Synthesis, structure and properties of N-alkoxy-N-(1-pyridinium)urea salts, N-alkoxy-N-acyloxyureas and N,N-dialkoxyureas. Mendeleev Commun., 17(3), 178–180. https://doi.org/10.1016/j.mencom.2007.05.016
Shishkin, O.V., Shtamburg, V.G., Zubatyuk R.I., Olefir, D.A., Tsygankov, A.V., Prosyanik, A.V., Mazepa,A.V., Kostyanovsky, R.G. (2009). Chiral Ureas with Two Electronegative Substituents at 1-N and Unusual Case of Coexisting a Pyramidal and Almost Planar 1-N in The Same Crystal. Chirality, 21(7). 642–647. https://doi.org/10.1002/chir.20668
Shtamburg, V.G., Kostyanovsky, R.G., Tsygankov, A.V., Shtamburg, V.V., Shishkin, O.V., Zubatyuk, R.I., Mazepa, A.V., Kravchenko, S.V. (2015). Geminal Systems. Communication 64. N-Alkoxy-N-chloroureas and N,N-Dialkoxyureas, Russ. Chem. Bulletin. Intern. Ed., 64(1), 62–75. https://doi.org/10.1007/s11172-015-0822-9
Shtamburg, V.G., Tsygankov, A.V., Gerasimenko, M.V., Shishkin, O.V., Zubatyuk, R.I., Mazepa, A.V.; Kostyanovsky, R.G. (2011). New approach to N,N-dialkoxy-N'-arylureas and N,N-dialkoxycarbamates. Mendeleev Commun., 21(12), 50−32. https://doi.org/10.1016/j.mencom.2011.01.021
Shtamburg, V.G., Tsygankov, A.V., Shishkin, O.V., Zubatyuk, R. I., Shtamburg, V.V., Gerasimenko, M.V., Mazepa, A.V., Kostyanovsky, R.G. (2012). 1-Alkoxyamino-4-dimethylaminopyridinium derivatives as new representatives of O-N-N+geminal systems and their structure. Mendeleev Commun., 22(2), 92−94. https://doi.org/10.1016/j.men.com.2012.02.014
Shtamburg, V.G., Shishkin, O.V., Zubatyuk, R.I., Shtamburg, V.V., Tsygankov, A.V., Mazepa, A.V., Kadorkina, G.K., Kostyanovsky, R.G. (2013). Synthesis and structure of N-alkoxyhydrazines and N-alkoxy-N’,N’,N-trialkylhydrazinium salts, Mendeleev Commun., 23(5), 289−291. https://doi.org./10.1016/j.men.com.2013.09.018
Shtamburg, V.G., Shtamburg, V.V., Tsygankov, A.V., Anishchenko, A.A., Zubatyuk, R.I., Shishkina, S.V., Mazepa, A.V., Klots, E.A. (2016). Synthesis and Structure of New N-Alkoxy-N-(1-pyridinium)urea Chlorides. Eur. Chem. Bull., 5(4), 142–146. doi: 10.17628/ECB.2016.5.142
Shtamburg, V.G., Klots, E.A., Pleshkova, AP., Avramenko, V.I., Ivonin, S.P., Tsygankov, A.V, Kostyanovsky, R.G. (2003). Geminal systems. 50. Synthesis and alcoholysis on N-acyloxy-N-alkoxy derivatives of ureas, carbamates and benzamide. Russ. Chem. Bull., Int. Ed., 52 2251−2260. https://doi.org./10.1023/B:RUCB.0000011887.405.29.b0
Shtamburg, V.G., Shtamburg, V.V., Kravchenko, S.V., Mazepa, A.V., Anishchenko, A.A., Posokhov, E.A. (2017). A New Synthesis of N-Alkoxyaminopyridinium Salts. Bulletin of National University “KhPI”. Series: New solutions in modern technology, 7, 211−218. https://doi.org/10.20998/2413−4295.2017.07.30
Shtamburg, V.G., Anishchenko, A.A., Shishkina, S.V., Konovalova, I.S., Shtamburg, V.V., Mazepa, A.V., Kravchenko, S.V. (2017). 1-(N-Ethoxycarbonyl-N-isopropyloxy)amino-4-dimethylaminopyridinium Chloride. Synthesis and Structure. Eur. Chem. Bull., 6(10), 470–474. https://doi.org/10.17628/ecb.2017.6.470-474
Sheldrick, G.M. (2008). A short history of SHELX. Acta Cryst., Sect. A., A64, 112−122. https://doi.org/10.1107/S0108767307043930
Mai, X., Xia, H.-Y., Cao Y.-S., Lu X.-S., Liao, Y.-J. (2009). Crystal structure of 1-(benzyloxy)urea, C8H10N2O2. Z. Kristallogr. NCS, 224, 547−548. https://doi.org/10.1524/ncrs.2009.0238
Burgi, H.-B., Dunitz, J.D. (1994). VCH. Weinheim., Structure correlation, 2, 741−784. https://doi.org./10.1107/S0108768195009931
Glover, S. A., White, J. M., Rosser, A. A., Digianantonio, K.M. (2011). Structure of N,N-Dialkoxyamides: Pyramidal Anomeric Amides with Low Amidicity. J. Org. Chem., 76(23), 9757−9763. https://doi.org/10.1021/jo201856u
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

