INFLUENCE OF ELECTRO-CATALYSIS ON EMISSIONS TO THE ENVIRONMENT DURING SOLID FUEL COMBUSTION
DOI:
https://doi.org/10.15421/jchemtech.v32i2.296301Keywords:
electrocatalysis; burning; nitrogen oxides; carbon monoxides;dielectric barrier dischargeAbstract
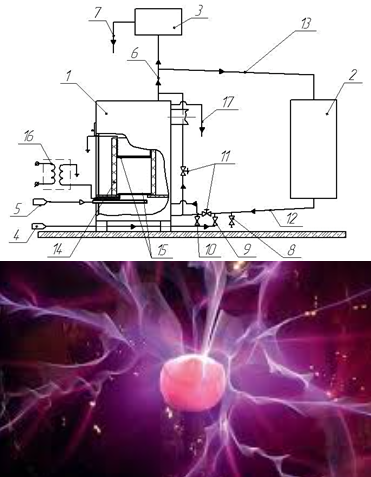
Nitrogen oxides, carbon oxides, and a large amount of various slags are among the largest emissions into the environment during the burning of solid fuel. Now there are three main sources of nitrogen oxide formation: "thermal", "fast" and "fuel". Each of the sources of formation of nitrogen oxides has its own formation mechanism. Carbon oxides (II) are formed during incomplete oxidation of fuel carbon along with aldehydes, organic acids and other hydrocarbons. One of the options for reducing atmospheric emissions and increasing the degree of solid fuel combustion is the use of electrocatalysis, the essence of which is to intensify the primary endothermic stages of the solid fuel combustion reaction, which are based on the use of directed action of an artificially created low-temperature plasma with an ordered movement of "slow" electrons. A dielectric barrier discharge was used as a source of "slow" electrons. With electro-catalytic combustion, a decrease in the concentration of nitrogen oxide (II) reaches almost 49 %, and carbon monoxide, at the same voltage, almost 33 %. Fuel burn rate increased by 30 %. The decrease in the formation of nitrogen oxides (II) can be explained by the fact that when using electrocatalysis, the formation of "thermal" and “fuel” nitrogen oxides is suppressed due to the fixation of atomic oxygen. Suppression of the formation of fast nitrogen oxides (II) occurs due to an increase in the thermal effect of the ongoing process.
Carbon (II) oxides are also oxidized to carbon (IV) oxide due to better diffusion of oxygen and the flow of "slow" electrons and an increase in the number of radicals HO•.
References
Grimsberg, M. (1990). Formation of nitrogen oxides during combustion. Sweden: N.
Asghar, U., Rafiq, S., Anwar, A., Iqbal, T., Ahmed, A., Jamil, F., M., Khurram, S., Akbar, M.M., Farooq, A., Shah, N.S., Park, Y-K. (2021). Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. Journal of Environmental Chemical Engineering, 9(5), 106064 https://doi.org/10.1016/j.jece.2021.106064
Cellek, М.S. (2022). The decreasing effect of ammonia enrichment on the combustion emission of hydrogen, methane, and propane fuels. International Journal of Hydrogen Energy, 47(45), 19916–19934. https://doi.org/10.1016/j.ijhydene.2021.11.241.
Zajemska, M., Poskart, A., Musiał, D. (2015). The kinetics of nitrogen oxides formation in the flame gas. Economic and Environmental Studies, 15(4), 444-461.
Gao, S., Zhang, X., Chen, L., Cui, Y., Jiang, J., Zhang, Z., Peifeng, Yu., Wang, C. (2022). Review: Radiation temperature measurement methods for engine turbine blades and environment influence Infrared Physics & Technology, 123, 104204.
https://doi: 10.1016/j.infrared.2022.104204.
Anetor, L., Odetunde, C. Osakue, E. E. (2014). Computational Analysis of the Extended Zeldovich Mechanism. Arab J Sci Eng 39, 8287–8305. https://doi.org/10.1007/s13369-014-1398-7
Godin, J., Liu, W., Ren, S., Xu, C. C. (2021). Advances in recovery and utilization of carbon dioxide: A brief review. Journal of Environmental Chemical Engineering, 9, 105644 https://doi.org/10.1016/j.jece.2021.105644
Karlström, O., Perander, M., DeMartini, N., Brink, A., Hupa, M. (2017). Role of ash on the NO formation during char oxidation of biomass. Fuel, 190, 274–280. https://doi.org/10.1016/j.fuel.2016.11.013
Zhou, H., Li, Y., Li, N., Qiu, R., Sheng Meng, S., Cen, K. (2017). Experimental study of the NO and N2O emissions during devolatilization and char combustion of a single biomass particle in O2/N2 and O2/H2O under low temperature condition. Fuel, 206, 162–170 https://doi.org/10.1016/j.fuel.2017.05.089
Turgut M. Gür, T. M. (2022). Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Progress in Energy and Combustion Science, 89, 100965. https://doi.org/10.1016/j.pecs.2021.100965
Paraschiv, L. S., Serban, A., Paraschiv, S. (2020). Calculation of combustion air required for burning solid fuels (coal /biomass / solid waste) and analysis of fl ue gas composition. Energy, 6, 36–45. https://doi.org/10.1016/j.egyr.2019.10.016
Ollegott, K., Wirth, Ph., Oberste-Beulmann, C., Awakowicz, P., Muhler., M (2020). Fundamental Properties and Applications of Dielectric Barrier Discharges in Plasma-Catalytic Processes at Atmospheric Pressure, Chem. Ing. Tech., 92(10), 1542–1558. https://doi.org/10.1002/cite.202000075
Kogelschatz, U. (2003). Dielectric-barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chemistry and Plasma Processing, 23(1), 1–46. https://doi.org/10.1023/A:1022470901385
Sharma, N.K, Misra, S, Varun, V, Pal, U. N. (2020). Experimental and simulation analysis of dielectric barrier discharge based pulsed cold atmospheric pressure plasma jet Physics of Plasmas 27, 113502. https://doi.org/10.1063/5.0018901
Lacoste, D.A. (2023). Flames with plasmas. Proceedings of the Combustion Institute, 39(4), 405–5428 https://doi.org/10.1016/j.proci.2022.06.025
He, J., Wen, X., Wu, L., Chen, H. (2022). Dielectric barrier discharge plasma for nanomaterials: Fabrication, modification and analytical applications. TrAC Trends in Analytical Chemistry, 156. https://doi.org/10.1016/j.trac.2022.116715
Li J., Ma, C., Zhu, S., Yu, F., Dai, B., Yang, D. (2019). A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials, 9, 1428. https://doi.org/10.3390/nano9101428
Uytdenhouwen, Y., Bal, K. M., Neyts, E. C., Meynen, V., Cool, P., Bogaerts, A. (2021). On the kinetics and equilibria of plasma-based dry reforming of methane. Chemical Engineering Journal, 405(1), 126630. https://doi.org/10.1016/j.cej.2020.126630
Bogaerts, A., Zhang, Q. Z., Zhang, Y. R. , Laer, K. V., Wang, W. (2019). Burning questions of plasma catalysis: Answers by modelling. Catalysis Today, 337, 15. https://doi.org/10.1016/j.cattod.2019.04.077
Nguyen-Kuok, S (2017). [Theory of Low-Temperature Plasma Physics], Springer, SSAOPP, vol. 95.
Engeln, R., Klarenaar, B., Guaitella, O. (2020). Foundations of optical diagnostics in low-temperature plasmas. Plasma Sources Sci. Technol., 29, 063001 https://doi.org/10.1088/1361-6595/ab6880
Vіazovyk, V. (2023). [Electrocatalytic intensification of solid fuel combustion]. Journal of Chemistry and Technologies, 31(3), 572–580. https://doi.org/10.15421/jchemtech.v31i3.285955 (in Ukrainian)
Viazovyk, V., Stolyarenko, H., Vodianik, O. (2011) The alternative burning of coal. Nauka i studia, Chemia. 110–115.
Vyazovyk, V. M. (2023). [Electron-catalytic intensification of the mountain of gas-like fire]. Journal of Chemistry and Technologies, 31(1), 186–194. https://doi.org/10.15421/jchemtech.v31i1.271226 (in Ukrainian)
Warnatz, Y., Maas, W., Dibble, R. (2012). Combustion: Physical and Chemical Fundamentals, Modeling and Simulation, Experiments, Pollutant Formation, Springer Science & Business Media
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

