STUDY OF VANADATE UNITS USING DENSITY FUNCTIONAL THEORY: ELECTRONIC PROPERTIES AND REACTIVITY
DOI:
https://doi.org/10.15421/jchemtech.v32i2.296585Keywords:
Vanadate units; DFT; Global reactivity indices; electrostatic potential; nucleophilic; FUKUI indexes.Abstract
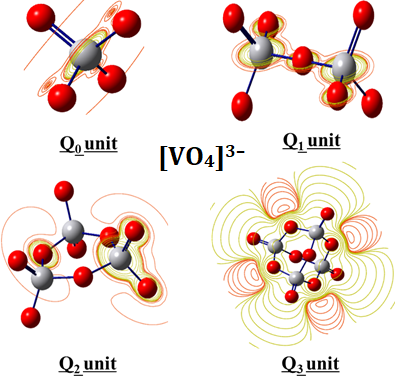
The vanadate units have been theoretically investigated through density functional theory calculations. The global reactivity indices have been optimized. The obtained results revealed that the (Q0) [VO4]3– unit shows an electron-donor character, while the units Q1, Q2, and Q3 units are evidenced to exhibit an electron-acceptor feature. The transition from one unit to another is found to be accompanied by an increase in the number of bridging oxygen atoms, in accordance with the highlighted changes in Mulliken charges. Moreover, the analysis of the optimized electrostatic potential surfaces indicated a higher likelihood of nucleophilic attacks on the vanadium atoms. Predictions for infrared and Raman spectra were also conducted, revealing changes in symmetric and asymmetric vibrational bands as the number of bridging oxygen atoms varied. Additionally, Fukui indices were employed to identify the preferred sites for the electrophilic attack within the (Q0) [VO4]3– unit on the Q1, Q2, and Q3 vanadate units.
References
Lee, J. C., Kim, E. Y., Chung, K. W., Kim, R. H., Jeon, S. (2021). A review on the metallurgical recycling of vanadium from slags: towards a sustainable vanadium production. J. Mater. Res. Technol., 12, 343–364. https://doi.org/10.1016/j.jmrt.2021.02.065
Wachs, I. E. (2013). Catalysis science of supported vanadium oxide catalysts. Dalton Trans., 42(33), 11762–11769.
Lourenssen, K., Williams, J., Ahmadpour, F., Clemmer, R., Tasnim, S. (2019). Vanadium redox flow batteries: A comprehensive review. J. Energy Storage, 25, 100844. https://doi.org/10.1016/j.est.2019.100844
Liu, K. Lee, S. Yang, S., Delaire, O., Wu, J. (2018). Recent progresses on physics and applications of vanadium dioxide. Mater. Today, 21(8), 875–896. https://doi.org/10.1016/j.mattod.2018.03.029
Muroga, T., Chen, J. M., Chernov, V. M., Kurtz, R. J., Le Flem, M. (2014). Present status of vanadium alloys for fusion applications. J. Nucl. Mater. 455(1-3), 263–268.
Scibior, A., Pietrzyk, L., Plewa, Z., Skiba, A, (2020). Vanadium: risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends, Journal of Trace Elements in Medicine and Biology. 61, 126508. https://doi.org/10.1016/j.jtemb.2020.126508
Menictas, C., Skyllas-Kazacos, M. (2011). Performance of vanadium-oxygen redox fuel cell. J. Appl. Electrochem. 41, 1223–1232.
Skyllas‐Kazacos, M., Kazacos, G., Poon, G., Verseema, H. (2010). Recent advances with UNSW vanadium‐based redox flow batteries. Int. J. Energy Res. 34(2), 182–189.
Webster, S., Czerw, R., Nesper, R., DiMaio, J., Xu, J. F., Ballato J., Carroll, D. L. (2004). Optical properties of vanadium oxide nanotubes. J. Nanosci. Nanotechnol. 4(3), 260–264.
doi: 10.1166/jnn.2004.035
Sindhu, S., Sanghi, S. A. Agarwal, Seth Sonam, V. P. Kishore, N. (2005). The role of V2O5 in the modification of structural, optical and electrical properties of vanadium barium borate glasses. Phys. B: Condens. Matter. 365, 65.
Lakshmikantha, R., Ayachit, N.H., Anavekar, R. V. (2014). Optical, physical and structural studies of vanadium doped P2O5–BaO–Li2O glasses, Journal of Physics and Chemistry of Solids 75 168–173.
Khattak, G. D., Tabet, N., Wenger, L. E. (2005). Structural properties of glasses in the series (SrO)x(V2O5)1−x, (SrO)0.5−y(B2O3)y(V2O5)0.5, and (SrO)0.2(B2O3)z(V2O5)0.8−z. Phys. Rev. B, 72, 104203.
Brow, R. K. Non- Cryst, J. (2000). The structure of simple phosphate glasses. Solids, 263-264, 1–28.
Ehrt, D. (2015). Phosphate and fluoride phosphate optical glasses — properties, structure and applications, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol. B, 56(6), 217–234.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman J. R. (2009). Gaussian 09 revision C, 1. Gaussian Inc, Wallingford C.T.
Khan, M. F. R., Rashid, B., Rahman, M. M., Al Faruk, M. M., Rahman, M., Rashid, M. A. (2017). Effects of solvent polarity on solvation free energy, dipole moment, polarizability, hyperpolarizability and molecular reactivity of aspirin. Int. J. Pharm. Pharm. Sci, 9(2), 217–221
Vijayakumar, S., Kolandaivel, P. (2006). Study of static dipole polarizabilities, dipole moments, and chemical hardness for linear CH3–(CC) n–X (X= H, F, Cl, Br, and NO2 and n= 1–4) molecules. J. Mol. Struct. Theochem. 770(1-3), 23–3.
Afzal, Q. Q., Jaffar, K., Ans, M., Rafique, J., Iqbal, J., Shehzad, R. A., Mahr, M. S. (2022). Designing benzothiadiazole based highly efficient non-fullerene acceptor molecules for organic solar cells. Polymer, 238, 124405. https://doi.org/10.1016/j.polymer.2021.124405
Valero-Romero, M. J. Cabrera-Molina, A. Gomero-Pérez, M. O Rodrigez-Mirasol J., Cordero, T. (2014). Catalysis Today, 233–241.
Dasireddy, V. D. B. C., Singh S., Friedrich, H. B. (2012). Applied Catalysis A: General, 421-422, 58–69.
Cheng, L. Ferguson, G. L. Zygmunt S. A., Curtiss, L. A. (2013). Journal of Catalysis, 302, 31–36.
Buyukuslu, H., Akdogan, M., Yildirim, G., Parlak, C. (2010). Ab initio Hartree-Fock and density functional theory study on characterization of 3-(5-methylthiazol-2-yldiazenyl)-2-phenyl-1H-indole. Spectrochim. Acta A, 75, 1362.
Parr, R., Szentpaly, L., Liu, S. (1999). Electrophilicity index. J. Am. Chem. Soc., (121) 1922.
Lipkowitz, K. B., Boyd, D. B. (2009). Reviews in Computational Chemistry, Vol. 5, John Wiley & Sons.
Naray-Szabo, G., Ferenczy, G. (1995). Molecular electrostatics. Chem. Rev. 95, 829.
Nataraj, A., Balachandran, V., Karthick, T. (2013). Molecular orbital studies (hardness, chemical potential, electrophilicity, and first electron excitation), vibrational investigation and theoretical NBO analysis of 2-hydroxy-5-bromobenzaldehyde by density functional method. J. Mol. Struct., 1031, 221–233.
Guo, H. W., Wang, X. F., Gong, Y. X., Gao D. N. (2010). Mixed alkali effect in xK2O-(30−x) Na2O-30P2O5-40ZnO glasses. J Non Cryst Solids. 356, 2109–2113.
Ternane, R., Ferid, M., Guyot, Y. Malika, T. A., Boulon, G. (2008). Spectroscopic properties of Yb3+ in NaYbP2O7 diphosphate single crystals. J Alloys Comp., 464, 327–331.
Abid, M., Et-tabirou, M., Hafid, M. (2001). Glass forming region, ionic conductivity and infrared spectroscopy of vitreous sodium lead mixed phosphates. Mater. Res. Bull., 36, 407–421.
Bet-thet, P., Bretey, E., Berthon J., d’Yvoire, F., Belkebir, A., Rulmont, A., Gilbert, B. (1994). Structure and ion transport properties of Na2O Ga2O3 P2O5 glasses. Solid State Ionics, 70/71, 476–481.
Mandlule, A., Döhler, F., VanWüllen, L., Kasuga, T., Brauer, D. S. (2014). Changes in structure and thermal properties with phosphate content of ternary calcium sodium phosphate glasses. J. Non Cryst. Solids, 392–393, 31–38.
Vanasundari, K., Balachandran, V., Kavimani, M., Narayana, B. (2017). Spectroscopic investigation, vibrational assignments, Fukui functions, HOMO-LUMO, MEP and molecular docking evaluation of 4 – [(3, 4 – dichlorophenyl) amino] 2 – methylidene 4 – oxo butanoic acid by DFT method, Journal of Molecular Structure, 1147, 136–147.
Haldhar, R., Prasad, D., Mandal, N., Benhiba, F., Bahadur, I., Dagdag, O. (2021). Anticorrosive properties of a green and sustainable inhibitor from leaves extract of Cannabis sativa plant: Experimental and theoretical approach. Colloids Surf. A: Physicochem. Eng. 614, 126211. https://doi.org/10.1016/j.colsurfa.2021.126211
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

