SPECTRAL CHARACTERISTICS OF PROTOCYANIN SUPRAMOLECULAR PIGMENT AS PHARMACOGNOSTIC CRITERIA OF CENTAUREA CYANUS L. FLOWERS
DOI:
https://doi.org/10.15421/jchemtech.v32i2.298075Keywords:
protocyanin, cornflower petals, anthocyanins, copigmentation, reflectance spectra, colorimetryAbstract
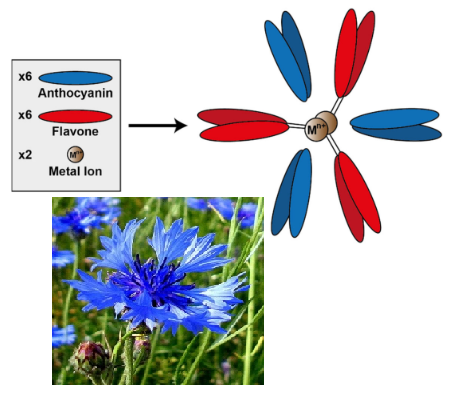
The results of the study of cornflower (Centaurea cyanus L.) petals by the spectrophotometric method are presented. It has been established that a set of spectral characteristics of the supramolecular pigment complex of protocyanin in the petals can be used for the pharmacognostic analysis of medicinal raw materials: the presence of a long-wavelength band with distributed maxima in the reflectance spectra and a color stimulus with a dominant wavelength in the blue range. The identified diagnostic features distinguish the type with blue color as the main object in the preparation of raw materials of the official medicinal plant from other different colored types under the conditions of intraspecific polychroism of flowers. Native protocyanin is obtained as a result of aqueous extraction of petals with a blue color. The appearance of maxima in the absorption spectrum of the aqueous blue extract corresponded to the characteristics of protocyanin in vivo in the reflectance spectra of petals of the type with a blue color. The difference in the spectral properties of the chromophoric systems of these forms is established by the parameters of the differential absorption spectrum of the solution of unassociated anthocyanin after acid destruction of the native pigment complex relative to protocyanin extract. Under the conditions of alcohol extraction of petals in an acidic environment, the destruction of pigment complexes, which are localized in surface tissues, and the formation of flavylium derivatives. In contrast to the reflectance spectra of multi-colored petals with a distributed band, a feature of the absorption spectra of their extracts is the presence of one maximum, the position of which depended on the type of coloring and indicated the dominance of pelargonidin or cyanidin glycosides. The obtained results improve the pharmacognostic criteria and can be used in the creation of biological preparations and functional food products based on raw cornflower.
References
Steed, J. W., Atwood, J. L. (2022). Supramolecular chemistry (3th ed.). Wiley.
Pina, F., Basílio, N., Parola, A. J., Melo, M. J., Oliveira, J., de Freitas, V. (2023). The triumph of the blue in nature and in Anthropocene. Dyes and Pigments, 210, 110925. https://doi.org/10.1016/j.dyepig.2022.110925
Houghton, A., Appelhagen, I., Martin, C. (2021). Natural blues: Structure meets function in anthocyanins. Plants, 10(4), 726. https://doi.org/10.3390/plants10040726
Yoshida, K., Mori, M., Kondo, T. (2009). Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat. Prod. Rep., 26(7), 884–915. https://doi.org/10.1039/B800165K.
Trouillas, P., Sancho-García, J. C., De Freitas, V., Gierschner, J., Otyepka, M., Dangles, O. (2016). Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev., 116, 4937–4982. https://doi.org/10.1021/acs.chemrev.5b00507.
Deng, C., Li, S., Feng, C., Hong, Y., Huang, H., Wang, J., Wang, L., Dai, S. (2019). Metabolite and gene expression analysis reveal the molecular mechanism for petal colour variation in six Centaurea cyanus cultivars. Plant Physiol. Biochem., 142, 22–33. https://doi.org/10.1016/j.plaphy.2019.06.018
Deng, C., Wang, J., Lu, C., Li, Y., Kong, D., Hong, Y., Huang, H., Dai, S. (2020). CcMYB6-1 and CcbHLH1, two novel transcription factors synergistically involved in regulating anthocyanin biosynthesis in cornflower. Plant Physiol. Biochem., 151, 271–283. https://doi.org/10.1016/j.plaphy.2020.03.024
Fedenko, V. S., Shemet, S. A., Landi, M. (2017). UV–vis spectroscopy and colorimetric models for detecting anthocyanin-metal complexes in plants: An overview of in vitro and in vivo techniques. J. Plant Physiol., 212, 13–28. https://doi.org/10.1016/j.jplph.2017.02.001
Upyr, L. V. (2010). [Cornflower. In Pharmaceutical encyclopedia] (2th ed.). Kyiv, Ukraine: MORION (in Ukrainian). https://www.pharmencyclopedia.com.ua/article/1798/voloshka
Kovalev, V. M., Marchyshin, S. M., Hvorost, O. P., Isakova, T. I. (Eds). (2014). Workshop on the identification of medicinal plant raw materials. Ternopil, Ukraine: TDMU (in Ukrainian).
Escher, G. B., Santos, J. S., Rosso, N. D., Marques, M. B., Azevedo, L., do Carmo, M. A. V., Daguerd, H., Molognonid, L., do Prado-Silvae, L., Sant'Anae, A. S., da Silva, M. C., Granato, D. (2018). Chemical study, antioxidant, anti-hypertensive, and cytotoxic/ cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem. Toxicol., 118, 439–453. https://doi.org/10.1016/j.fct.2018.05.046
Lockowandt, L., Pinela, J., Roriz, C. L., Pereira, C., Abreu, R. M., Calhelha, R. C., Alves, M. J., Barros, L. Bredol, M., Ferreira, I. C. (2019). Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod., 128, 496–503. https://doi.org/10.1016/j.indcrop.2018.11.059
Al-Snafi, A. E. (2015). The pharmacological importance of Centaurea cyanus – A review. Int. J. Pharm. Rev. Res., 5(4), 379–384.
Uçar, M. A., Derun, E. M., Pişkin, M. B. (2023). Determination of usage potential of Hypericum perforatum, Hypericum capitatum, Centaurea cyanus extracts and creams in the cosmetic industry. Sigma, 41(3), 443–450. https://doi.org/10.14744/sigma.2023.00051
Michalak, M. (2023). Plant extracts as skin care and therapeutic agents. Int. J. Mol. Sci., 24, 15444. https://doi.org/10.3390/ijms242015444
Papa, C. M., Suciu, A., Dopcea, I., Ene, N., Singh, S.K., Vamanu, E. (2023). Exploring the efficacy of extracts for cosmetic creams: In vivo and in vitro assessments. Nutraceuticals, 3, 306–314. https://doi.org/10.3390/nutraceuticals3030024
Pires Jr, E. D. O., Di Gioia, F., Rouphael, Y., García-Caparrós, P., Tzortzakis, N., Ferreira, I. C., Barros, L., Petropoulos, S.A., Caleja, C. (2023). Edible flowers as an emerging horticultural product: A review on sensorial properties, mineral and aroma profile. Trends Food Sci. Technol., 137, 31–54. https://doi.org/10.1016/j.tifs.2023.05.007
Teixeira, M., Tao, W., Fernandes, A., Faria, A., Ferreira, I. M., He, J., de Freitas, V., Mateus, N., Oliveira, H. (2023). Anthocyanin-rich edible flowers, current understanding of a potential new trend in dietary patterns. Trends Food Sci. Technol., 138, 708–725. https://doi.org/10.1016/j.tifs.2023.07.010
Janarny, G., Gunathilake, K. D. P. P., Ranaweera, K. K. D.S. (2021). Nutraceutical potential of dietary phytochemicals in edible flowers—A review. J. Food Biochem., 45(4), e13642. https://doi.org/10.1111/jfbc.13642
Kuś, P. M., Jerković, I., Tuberoso, C. I. G., Marijanović, Z., Congiu, F. (2014). Cornflower (Centaurea cyanus L.) honey quality parameters: Chromatographic fingerprints, chemical biomarkers, antioxidant capacity and others. Food Chem., 142, 12–18. https://doi.org/10.1016/j.foodchem.2013.07.050
Różyło, R., Szymańska-Chargot, M., Gawlik-Dziki, U., Dziki, D. (2021). Spectroscopic, mineral, and antioxidant characteristics of blue colored powders prepared from cornflower aqueous extracts. Food Chem., 346, 128889. https://doi.org/10.1016/j.foodchem.2020.128889
Vega, E. N., Ciudad-Mulero, M., Fernández-Ruiz, V., Barros, L., Morales, P. (2023). Natural sources of food colorants as potential substitutes for artificial additives. Foods, 12(22), 4102. https://doi.org/10.3390/foods12224102
Forschner, R., Knoeller, J. A., Zens, A., Frey, W., Molard, Y., Laschat, S. (2023). Luminescent liquid crystals: from supramolecular plant dyes to emissive flavylium salts. Liq. Cryst., 50(7-10), 1310–1323. https://doi.org/10.1080/02678292.2023.2179122
Jaison, J. P., Balasubramanian, B., Gangwar, J., James, N., Pappuswamy, M., Anand, A. V., Al-Dhabi, N. A., Arasu, M. V., Liu, W.-C., Sebastian, J. K. (2023). Green synthesis of bioinspired nanoparticles mediated from plant extracts of Asteraceae family for potential biological applications. Antibiotics, 12(3), 543. https://doi.org/10.3390/antibiotics12030543
Hlushchenko, L. A., Kutsenko, N. I. (2023). Problems with identification of medicinal plants and medicinal plant raw materials. J. Native Alien Plant Stud., 19, 38–52 (in Ukrainian). https://doi.org/10.37555/2707-3114.19.2023.293647
Fedenko, V. S. (2006). [Cyanidin complexation with metal ions]. Ukrains' kyi Biokhimichnyi Zhurnal, 78(2), 149–152 (in Ukrainian).
Fedenko, V. S. (2007). [Dose effect of cyanidin interaction with lead ions in roots of maize seedlings]. Ukrains' kyi Biokhimichnyi Zhurnal, 79(2), 24–29 (in Ukrainian).
Fedenko, V. S. (2008). [Cyanidin as endogenous chelator of metal ions in maize seedling roots]. Ukrains' kyi Biokhimichnyi Zhurnal, 80(1), 102–106 (in Ukrainian).
Fedenko, V. S., Landi, M., Shemet, S. A. (2017). Detection of nickel in maize roots: A novel nondestructive approach by reflectance spectroscopy and colorimetric models. Ecol. Indic., 82, 463–469. https://doi.org/10.1016/j.ecolind.2017.07.021
Fedenko, V. S. (2022). Chemisorption of flavonoids from canadian goldenrod on aluminum oxide. J. Chem. Technol., 30(3), 340–348 (in Ukrainian). https://doi.org/10.15421/jchemtech.v30i3.262972
Newsome, A. G., Culver, C. A., Van Breemen, R. B. (2014). Nature’s palette: the search for natural blue colorants. J. Agric Food Chem., 62(28), 6498–6511. https://doi.org/10.1021/jf501419q
Haratym, W., Weryszko-Chmielewska, E., Konarska, A. (2020). Microstructural and histochemical analysis of aboveground organs of Centaurea cyanus used in herbal medicine. Protoplasma, 257, 285–298. https://doi.org/10.1007/s00709-019-01437
Brangule, A., Šukele, R., Bandere, D. (2020). Herbal medicine characterization perspectives using advanced FTIR sample techniques–diffuse reflectance (DRIFT) and photoacoustic spectroscopy (PAS). Front. Plant Sci., 11, 356. https://doi.org/10.3389/fpls.2020.00356
Różyło, R., Szymańska-Chargot, M., Zdunek, A., Gawlik-Dziki, U., Dziki, D. (2022). Microencapsulated red powders from cornflower extract—spectral (FT-IR and FT-Raman) and antioxidant characteristics. Molecules, 27(10), 3094. https://doi.org/10.3390/molecules27103094
Żbik, K., Onopiuk, A., Szpicer, A., Kurek, M. (2023). Comparison of the effects of extraction method and solvents on biological activities of phytochemicals from selected violet and blue pigmented flowers. J. Food Meas. Charact., 17(6), 6600–6608. https://doi.org/10.1007/s11694-023-02158-2
Bruni, S., Longoni, M., Minzoni, C., Basili, M., Zocca, I., Pieraccini, S., Sironi, M. (2023). Resonance Raman and visible micro-spectroscopy for the in-vivo and in-vitro characterization of anthocyanin-based pigments in blue and violet flowers: A Comparison with HPLC-ESI-MS analysis of the extracts. Molecules, 28(4), 1709. https://doi.org/10.3390/molecules28041709
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

