STUDY OF THE INFLUENCE OF SYNTHESIS PARAMETERS ON THE STRUCTURAL AND MAGNETIC PROPERTIES OF COBALT FERRITE
DOI:
https://doi.org/10.15421/jchemtech.v32i1.298377Keywords:
cobalt ferrite, plasma chemical synthesis, X-ray phase analysis, experimental planning, saturation magnetization, crystallite sizeAbstract
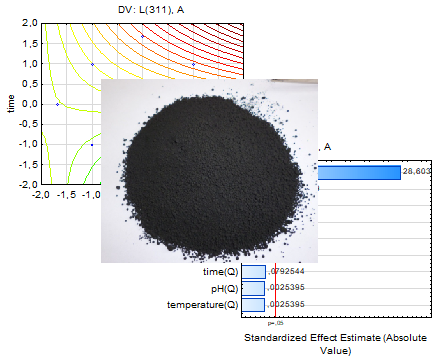
The synthesis of cobalt ferrite by plasma chemical method was studied in the work. The influence of pH of the reaction medium, temperature and duration of treatment on magnetic properties, intensity of peaks on X-ray diffraction, average size of crystallites, value of dislocation density was determined using the central composite rotatable planning of the experiment, which is based on the results obtained by X-ray phase analysis, vibrational magnetometry, and electron microscopy. Statistical analysis made it possible to quantitatively assess the influence of the synthesis parameters on the selected response functions. The results showed that the pH of the reaction medium is the parameter that shows the greatest influence both on the growth of cobalt ferrite powder crystallites and on the magnetic properties of the samples obtained by the plasma chemical method.
References
Liandi, A. R., Cahyana, A. H., Kusumah, A. J. F., Lupitasari, A., Alfariza, D. N., Nuraini, R., Sari, R. W., Kusumasari, F. C. (2023). Recent trends of spinel ferrites (MFe2O4: Mn, Co, Ni, Cu, Zn) applications as an environmentally friendly catalyst in multicomponent reactions: A review. Case Studies in Chemical and Environmental Engineering, 7, 100303. https://doi.org/10.1016/j.cscee.2023.100303
Frolova, L. A., Khmelenko, O. V. (2021). The study of Co–Ni–Mn ferrites for the catalytic decomposition of 4-nitrophenol. Catalysis Letters, 151, 1522–1533. https://doi.org/10.1007/s10562-020-03419-1
Rezaei, B., Yari, P., Sanders, S. M., Wang, H., Chugh, V. K., Liang, S., Shahriar Mostufa, S., Xu, K., Wang, J. P., Gómez-Pastora, J., Wu, K. (2023). Magnetic nanoparticles: a review on synthesis, characterization, functionalization, and biomedical applications. Small, 20(5), 2304848. https://doi.org/10.1002/smll.202304848
Bayça, F. (2024). Characterization and magnetic properties of CoFe2O4 nanoparticles synthesized by the co‐precipitation method. International Journal of Applied Ceramic Technology, 21(1), 544–554. https://doi.org/10.1111/ijac.14518
Yu, L., Fan, Y., Li, C., Liu, C., Ren, X., Yang, H., Lin, S. (2023). Synthesis and characterization of highly efficient oil–water separation, recyclable, magnetic particles CoFe2O4/SDB. Polymer Bulletin, 80(4), 3571–3584. doi:10.21203/rs.3.rs-601296/v1
de Medeiros, F., Madigou, V., Lopes-Moriyama, A. L., de Souza, C. P., Leroux, C. (2020). Synthesis of CoFe2O4 nanocubes. Nano-Structures & Nano-Objects, 21, 100422. doi:10.1016/j.nanoso.2019.100422
Frolova, L., Khmelenko, O. (2018). Investigation of the magnetic properties of ferrites in the CoO-NiO-ZnO using simplex-lattice design. Journal of Nanomaterials, 2018, 1–8. https://doi.org/10.1155/2018/5686741
Malinowska, I., Ryżyńska, Z., Mrotek, E., Klimczuk, T., Zielińska-Jurek, A. (2020). Synthesis of CoFe2O4 nanoparticles: the effect of ionic strength, concentration, and precursor type on morphology and magnetic properties. Journal of Nanomaterials, 2020, 1–12. https://doi.org/10.1155/2020/9046219
Wei, K., Huai, H. X., Zhao, B., Zheng, J., Gao, G. Q., Zheng, X. Y., Wang, C. C. (2022). Facile synthesis of CoFe2O4 nanoparticles and their gas sensing properties. Sensors and Actuators B: Chemical, 369, 132279. http://dx.doi.org/10.1016/j.snb.2022.132279
Alharthy, R. D., Saleh, A. (2021). A novel trace-level ammonia gas sensing based on flexible PAni-CoFe2O4 nanocomposite film at room temperature. Polymers, 13(18), 3077. https://doi.org/10.3390/polym13183077
Chen, K., Li, Y., Du, Z., Hu, S., Huang, J., Shi, Z., Su, B., Yang, G. (2022). CoFe2O4 embedded bacterial cellulose for flexible, biodegradable, and self-powered electromagnetic sensor. Nano Energy, 102, 107740. DOI:10.2139/ssrn.4136294
Ansari, M. A., Govindasamy, R., Begum, M. Y., Ghazwani, M., Alqahtani, A., Alomary, M. N., Jamous, Y. F., Alyahya, S. A., Asiri, S., Khan, F. A., Almessiere, M. A., Baykal, A. (2023). Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications. Nanotechnology Reviews, 12(1), 20230575. doi:10.1515/ntrev-2023-0575
Abimathi, N., Harshene, H., Vidhya, B. (2022). Synthesis and characterization of CoFe2O4 nanoparticles with its medical application. Materials Today: Proceedings, 62(1-2), 2315–2319. doi:10.1016/j.matpr.2022.04.101
Khizar, S., Ahmad, N. M., Ahmed, N., Manzoor, S., Hamayun, M. A., Naseer, N., Tenorio, M.K.L., Lebaz, N., Elaissari, A. (2020). Aminodextran coated CoFe2O4 nanoparticles for combined magnetic resonance imaging and hyperthermia. Nanomaterials, 10(11), 2182. https://doi.org/10.3390/nano10112182
Frolova, L., Sukhyy, K. (2022). The effect of the cation in spinel ferrite MeFe2O4 (Me = Co, Ni, Mn) on the photocatalytic properties in the degradation of methylene blue. Materials Today: Proceedings, 62, 7726–7730. https://doi.org/10.1016/j.matpr.2022.03.503
Frolova, L. (2020). Photocatalytic activity of spinel ferrites CoxFe3−xO4 (0.25< x< 1) obtained by treatment contact low-temperature non-equilibrium plasma liquors. Applied Nanoscience, 10(12), 4585. doi:10.1007/s13204-020-01344-8
Sun, Q., Wu, S., Li, K., Han, B., Chen, Y., Pang, B., Yu, L., Dong, L. (2020). The favourable synergistic operation of photocatalysis and catalytic oxygen reduction reaction by a novel heterogeneous CoFe2O4-TiO2 nanocomposite. Applied Surface Science, 516, 146142. DOI:10.1016/j.apsusc.2020.146142
Faroughi Niya, H., Hazeri, N., Fatahpour, M. (2021). Synthesis, characterization, and application of CoFe2O4@amino‐2‐naphthol‐4‐sulfonic acid as a novel and reusable catalyst for the synthesis of spirochromene derivatives. Applied Organometallic Chemistry, 35(3), e6119. https://doi.org/10.1002/aoc.6119
Sharifianjazi, F., Moradi, M., Parvin, N., Nemati, A., Rad, A. J., Sheysi, N., Abouchenari, A., Mohammadi, A., Karbasi, S., Ahmadi, Z., Khanian, A. E., Irani, M., Pakseresht, A., Sahmani, S., Asl, M. S. (2020). Magnetic CoFe2O4 nanoparticles doped with metal ions: a review. Ceramics International, 46(11), 18391–18412. doi:10.1016/j.ceramint.2020.04.202
Ji, G., Tang, S., Xu, B., Gu, B., Du, Y. (2003). Synthesis of CoFe2O4 nanowire arrays by sol–gel template method. Chemical physics letters, 379(5-6), 484–489. doi:10.1016/j.cplett.2003.08.090
Kim, Y. I., Kim, D., Lee, C. S. (2003). Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. Physica B: Condensed Matter, 337(1-4), 42–51. http://dx.doi.org/10.1016/S0921-4526(03)00322-3
Gyergyek, S., Makovec, D., Kodre, A., Arčon, I., Jagodič, M., Drofenik, M. (2010). Influence of synthesis method on structural and magnetic properties of cobalt ferrite nanoparticles. Journal of Nanoparticle Research, 12, 1263–1273. doi:10.1007/s11051-009-9833-5
Caldeira, L. E., Erhardt, C. S., Mariosi, F. R., Venturini, J., Zampiva, R. Y. S., Montedo, O. R. K., Arcaro, S., Bergmann C. P., Braganca, S. R. (2022). Correlation of synthesis parameters to the structural and magnetic properties of spinel cobalt ferrites (CoFe2O4)–an experimental and statistical study. Journal of Magnetism and Magnetic Materials, 550, 169128. doi:10.1016/j.jmmm.2022.169128
Lavorato, G., Alzamora, M., Contreras, C., Burlandy, G., Litterst, F. J., Baggio‐Saitovitch, E. (2019). Internal structure and magnetic properties in cobalt ferrite nanoparticles: Influence of the synthesis method. Particle & Particle Systems Characterization, 36(4), 1900061. https://doi.org/10.1002/ppsc.201900061
Senthil, V. P., Gajendiran, J., Raj, S. G., Shanmugavel, T., Kumar, G. R., Reddy, C. P. (2018). Study of structural and magnetic properties of cobalt ferrite (CoFe2O4) nanostructures. Chemical Physics Letters, 695, 19–23. http://dx.doi.org/10.1016/j.cplett.2018.01.057
Bououdina, M., Manoharan, C. (2020). Dependence of structure/morphology on electrical/magnetic properties of hydrothermally synthesised cobalt ferrite nanoparticles. Journal of Magnetism and Magnetic Materials, 493, 165703. doi:10.1016/j.jmmm.2019.165703
Purnama, B., Wijayanta, A. T., Suharyana, S. (2019). Effect of calcination temperature on structural and magnetic properties in cobalt ferrite nano particles. Journal of King Saud University-Science, 31(4), 956–960. doi:10.1016/j.jksus.2018.07.019
Frolova, L., Derimova, A., Khlopytskyi, A., Galivets, Y., & Savchenko, M. (2016). Investigation of phase formation in the system Fe2+/Co2+/O2/H2O. Eastern-European Journal of Enterprise Technologies, 6(6), 64–68. https://doi.org/10.15587/1729-4061.2016.85123
Frolova, L. A. (2014). Production conditions of iron oxide black from pickle liquors. Metallurgical & Mining Industry, (4), 65–69.
Ravindra, A. V., Ju, S. (2023). Mesoporous CoFe2O4 nanocrystals: Rapid microwave-hydrothermal synthesis and effect of synthesis temperature on properties. Materials Chemistry and Physics, 303(1-7), 127818. doi:10.1016/j.matchemphys.2023.127818
Frolova, L., Pivovarov, A., Tsepich, E. (2016). Non-equilibrium plasma-assisted hydrophaseferritization in Fе2+–Ni2+–SO42−–OH− System. Nanophysics, Nanophotonics, Surface Studies, and Applications. Springer Proceedingsin Physics, 183, 213–220. doi: 10.1007/978-3-319-30737-4_18
Frolova, L., Sukhyy, K. (2022). Investigation of the ferritization process in the Co 2+–Fe 2+–SO 4 2−–OH− system under the action of contact non-equilibrium low-temperature plasma. Applied Nanoscience, 1–8. https://doi.org/10.1007/s13204-021-01755-1
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

