SYNTHESIS N-ALKOXY-1-(DIMETHOXYPHOSPHORYLOXY)BENZIMIDATES FROM N-ALKOXY-N-CHLOROBENZAMIDES
DOI:
https://doi.org/10.15421/jchemtech.v32i3.298733Keywords:
N-alkoxy-N-chlorobenzamides, trimethyl phosphite, N-alkoxy-1-(dimethoxyphosphoryloxy)benzimidates, synthesis, structure, XRD study, N–O-migration of dimethoxyphosphoryl groupAbstract
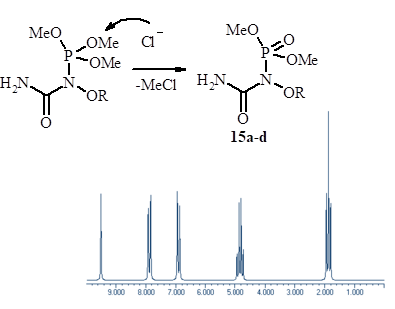
Aim. To synthesize of N-alkoxy-1-(dimethoxyphosphoryloxy)benzimidates from the interaction of N-alkoxy-N-chlorobenzamides with trimethylphosphite. To investigate N-alkoxy-1-(dimethoxyphosphoryloxy)-benzimidates structure using the XRD study. Methods. Mass spectrometry, 1H, 31P and 13C NMR spectroscopy, XRD study. Results. This study explores the reaction of N-alkoxy-N-chlorobenzamides with trimethylphosphite in ether resulting in the formation N-alkoxy-1-(dimethoxyphosphoryloxy)benzimidates. The obtained benzimidates are identified as the products of the nucleophilic substitution at nitrogen followed by an unusual N–O-migration of dimethoxyphosphoryl group. This reaction presents an original synthetic pathway to the N-alkoxy-1-phosphoryloxy imidates. In this research the possibility of the N-alkoxy-N-chlorobenzamides interaction with P-nucleophiles has been proved. The structure of N-alkoxy-1-(dimethoxyphosphoryloxy)benzimidates has been confirmed by the 1H, 31P and 13C NMR spectra, mass spectra and XRD study. The XRD study of N-methoxy-1-(dimethoxyphosphoryloxy)-4-nitrobenzimidate has demonstrated that this compound is Z-isomer, and 4-nitrophenyl moiety and N-methoxy group are in a trans position towards to the C=N double bond. The coplanarity of the aromatic ring and the π-system of the C=N double bond is evident from the XRD data. Conclusions. As the result of our study the feasibility of N-alkoxy-1-(phosphoryloxy)benzimidates formation through the interaction of N-alkoxy-N-chlorobenzamides with trialkylphosphites has been elucidated. This outcome holds significant value for a better understanding of the synthetic importance of N-alkoxy-N-chlorobenzamides. The structural elucidation of Z-N-alkoxy-1-(dimethoxyphosphoryloxy)benzimidates has been done. A novel kind of the intramolecular N–O-migration of the phosphoryl group has established.
References
Gerdes, R.G., Glover, S.A., ten Have, J.F., Rowbottom, C.A. (1989). N-Acetoxy-N-alkoxyamides – a New Class of Nitrenium Ion Precursors Which are Mutagenic. Tetrahedron Lett., 30(20), 2649−26521. https://doi.org/10.1016/S0040-4039(00)99089-0
Glover, S.A. (1998). Anomeric Amides – Structure, Properties and Reactivity. Tetrahedron, 54(26), 7229–7271. https://doi.org/10.1016/S0040-4020(98)00197-5
Gillson, A.-M., Glover, S.A., Tucker, D.J., Turner, P. (2003). Crystal structures and properties of mutagenic N-acyloxy-N-alkoxyamides – “most pyramidal” acyclic amides. Org. Biomol. Chem., 1(19), 3430−3437. https://doi.org/10.1039/B306098P
Glover, S.A. (2009). N-Heteroatom-substituted hydroxamic esters. in The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids, EdsRappoport, Z., Liebman, J. F., John Wiley and Sons, New York. PATAI’S Chemistry of Funcional Groups. v.1. https://doi.org/10.1002/9780470682531.pat0470
Glover, S.A., Rosser, A.A., (2018). Heteroatom Substitution at Amide Nitrogen – Resonance Reduction and HERON Reactions of Anomeric Amides. Molecules, 23(11), 2834. https://doi.org/10.3390/molecules23112834
Glover, S.A., Rosser, A.A. (2022). Modification of Amidic Resonance Through Heteroatom Substitution at Nitrogen: Anomeric Amides. Amide Bond Activation. Ed. M. Szostak, John Wiley and Sons, New York. https://doi.org/10.1002/9783527830251.ch2
Buccigross, J.M., Glover, S.A., Hammond, G.P. (1995). Decomposition of N,N’-Diacyl-N,N’-dialkoxyhydrazines Revisited. Aust. J. Chem. 48(2), 353−361. https://doi.org/10.1071/CH9950353
Glover, S.A., Mo, G. (2002). Hindered ester formation by SN2 azidation of N-acetoxy-N-alkoxyamides and N-alkoxy-N-chloroamides – novel application of HERON rearrangement. J. Chem. Soc., Perkin Trans, 2(10), 1728–1739. https://doi.org/10.1039/B111250N
Glover, S.A., Hammond, G.P., Bonin, A.M. (1998). A Comparison of the Reactivity and mutagenicity of N-(Benzoyloxy)-N-(benzyloxy)benzamides. J. Org. Chem., 63(26), 9684−9689. https://doi.org/10.1021/jo980863z
Cavanagh, K.L., Glover, S.A., Price, H.L., Schumacher, R.R. (2009). SN2 Substitution reactions at the Amide Nitrogen in the Anomeric Mutagens, N-Acyloxy-N-alkoxyamides. Aust. J. Chem., 62(7), 700–710. https://doi.org/10.1071/CH09166
Glover, S.A.; White, J.M.; Rosser, A.A.; Digianantonio, K.M. (2011). Structure of N,N-Dialkoxyamides: Pyramidal Anomeric Amides with Low Amidicity, J. Org. Chem., 76, 9757−9763. https://doi.org/10.1021/jo201856u
Shtamburg, V.G., Tsygankov, A.V., Shishkin, O.V., Zubatyuk, R.I., Uspensky, B.V., Shtamburg, V.V., Mazepa, A.V., Kostyanovsky, R.G. (2012). The properties and structure of N-chloro-N-methoxy-4-nitrobenzamide. Mendeleev Commun., 22(3), 164–166. https://doi.org/10.1016/j.mencom.2012.05.019
Shishkin, O.V., Zubatyuk, R.I., Shtamburg, V.G., Tsygankov, A.V., Klots, E.A., Mazepa, A.V., Kostyanovsky, R.G. (2006). Pyramidal Amide Nitrogen in N-Acyloxy-N-alkoxyureas and N-Acyloxy-N-alkoxycarbamates. Mendeleev Commun., 16 (4), 222−223. https://doi.org/10.1070/MC2006v016n04ABEH002195
Shtamburg, V.G., Shishkin, O.V., Zubatyuk, R.I., Kravchenko, S.V., Shtamburg, V.V., Distanov, V.B., Tsуgankov, A.V., Kostyanovsky, R.G. (2007). Synthesis, structure and properties of N-alkoxy-N-(1-pyridinium)urea salts, N-alkoxy-N-acyloxyureas and N,N-dialkoxyureas. Mendeleev Commun., 17(3), 178–180.https://doi.org/10.1016/j.mencom.2007.05.016
Shishkin, O.V., Shtamburg, V.G., Zubatyuk, R.I., Olefir, D.A., Tsygankov, A.V., Prosyanik, A.V., Mazepa, A.V., Kostyanovsky, R.G. (2009). Chiral Ureas with Two Electronegative Substituens at 1-N and Unusual Case of Coexisting a Pyramidal and Almost Planar 1-N in The Same Crystal. Chirality, 21(7), 642–647. https://doi.org/10.1002/chir.20668
Shtamburg, V.G., Tsygankov, A.V., Gerasimenko, M.V. ,Shishkin, O.V., Zubatyuk, R.I., Mazepa, A.V., Kostyanovsky, R.G. (2011). New approach to N,N-dialkoxy-N'-arylureas and N,N-dialkoxycarbamates. Mendeleev Commun., 21(12), 50−32. https://doi.org/10.1016/j.mencom.2011.01.021
Shtamburg, V.G., Shtamburg, V.V.,Tsygankov, A.V., Anishchenko, A.A., Zubatyuk, R.I. Shishkina, S.V., Mazepa, A.V., Klots, E.A. (2016). Synthesis and Structure of New N-Alkoxy-N-(1-pyridinium)urea Chlorides. Eur. Chem. Bull., 5(4), 142–146. doi: 10.17628/ECB.2016.5.142
Shtamburg, V.G., Shtamburg, V.V., Kravchenko, S.V., Mazepa, A.V., Anishchenko, A.A.,Posokhov, E.A. (2017). A New Synthesis of N-Alkoxyaminopyridinium Salts. Bulletin of National University “KhPI”. Series: New solutions in modern technology, (7), 211−218. doi:10.20998/2413-4295.2017.07.30
Shtamburg, V.G., Shishkin, O.V., Zubatyuk, R.I., Shtamburg V.V., Tsygankov, A.V., Mazepa, A.V., Kadorkina, G.K., Kostyanovsky, R.G. (2013). Synthesis and structure of N-alkoxyhydrazines and N-alkoxy-N’,N’,N-trialkylhydrazinium salts. Mendeleev Commun., 23(5), 289−291.https://doi.org./10.1016/j.men.com.2013.09.018
Shtamburg, V.G., Anishchenko, A.A., Shishkina, S.V., Konovalova, I.S., Shtamburg, V.V., Mazepa, A.V., Kravchenko, S.V. (2017). 1-(N-Ethoxycarbonyl-N-isopropyloxy)amino-4-dimethylaminopyridinium Chloride. Synthesis and Structure. Eur. Chem. Bull., 6 (10) (2017) 470–474. doi: 10.17628/ecb.2017.6.470-474
Shtamburg, V.G., Shishkina, S.V., Shtamburg, V.V., Mazepa, A.V., Kadorkina, G.K., Kostyanovsky, R.G. (2016). 1-Alkoxyamino-4-dimethylaminopyridinium salts: synthesis and structure. Mendeleev Commun., 26(2), 169−171. https://doi.org/10.1016/j.mencom.2016.03.030
Shtamburg, V.G., Shtamburg, V.V., Klots, E.A., Anishchenko, A.A., Mazepa, A.V., Kravchenko, S.V. (2020). Nucleophilic Substitution in N-Alkoxy-N-chlorocarbamates as a Way to N-Alkoxy-N’,N’,N’-trimethylhydrazinium chlorides. Eur. Chem. Bull., 9(1), 28−32. http://dx.doi.org/10.17628/ecb.2020.9.28-32
Shtamburg, V.G., Klots, E.A., Shtamburg, V.V., Anishchenko, A.A., Shishkina, S.V., Mazepa, A.V. (2023). Nucleophilic substitution at nitrogen atom. N-Alkoxy-N-(dimethoxyphosphoryl)ureas, synthesis and structure. J. Mol. Structure, 1277, 134865. https://doi.org/10.1016/j.molstruc.2022.134865
Sheldrick, G.M. (2008). A short history of SHELX. ActaCryst., Sect. A., A 64, 112−122. https://doi.org/10.1107/S0108767307043930
Plapinger, R.E., Wagner-Jauregg, T. (1953). A Nitrogen-to-Oxygen Phosphoryl Migration: Preparation of dl-Serinephosphoric and Threoninephosphoric Acid. J. Am. Chem. Soc., 75(22), 5757–5758. https://doi.org/10.1021/ja01118a524
Gao, X., Deng, H., Tang, G., Liu, Y., Xu, P., Zhao, Y. (2011). Intermolecular Phosphoryl Transfer of N-Phosphoryl Amino Acids. Eur. J. Org. Chem., 2011(17), 3220−3228. https://doi.org/10.1002/ejoc.201100234
Deng, X., Wang, Y., Liu, J.-B., Wan, C., Luo, N. (2022). Synthesis of N-methoxy-phosphoryloxyimidates through a copper-catalyzed cross-dehydrogenative coupling of N-methoxyamides with phosphites. Tetrahedron Lett. 105, 154049. https://ssrn.com/abstract=4150600
Zimin, M.G., Fomakhin, E.V., Sadykov, A.R., Smirnov, V.N., Pudovik, A.N. (1985). Synthesis and rearrangements of phosphorylated derivatives of phenyl isohydroxamic acid. Journal of General Chemistry of the USSR– Zhurnal Obshchei Khimii, 55(8), 1526−1531.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

