SYNTHESIS OF COPPER THIOSTIBIATE NANO COMPOUND BY SOLVOTHERMAL METHOD
DOI:
https://doi.org/10.15421/jchemtech.v32i2.299093Keywords:
solvothermal method, copper thiostibiate, nanocrystals, micromorphologyAbstract
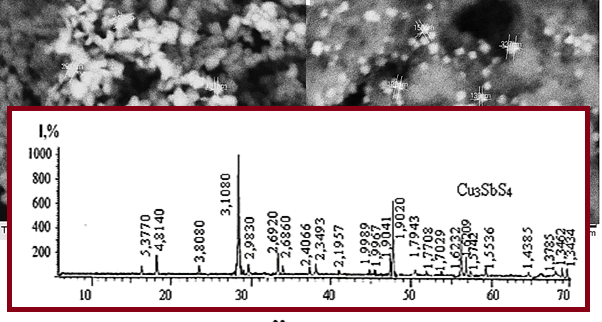
A simple and easily accessible synthesis of copper antimony sulfide nanocrystals is reported in a scientific study. The samples were synthesized using a solvothermal method with the help of a number of substances (copper, antimony, and elemental sulfur). During the experiments, potassium antimonyl tartrate was used as antimony material, and sulfur ethylenediamine solution was used as sulfide material. The synthesis of Cu3SbS4 nanoparticles was carried out by adjusting the molar ratio of sulfur to copper and controlling the volume ratios of the solvent and organic medium (ethylene glycol + polyethylene glycol). The process was carried out at a temperature of 383-393 K for 12 hours and copper-thiostibiate nanoparticles were synthesized with a yield of 85%. The properties of the obtained nanoparticles were studied by physical and chemical analysis methods - TGA, RFA, TEM, EDS.
References
An C, Jin Y, Tang K, Qian Y. Selective synthesis and characterization of famatinite nanofibers and tetrahedrite nanoflakes. Journal of Materials Chemistry. 2003;13(2):301-3.
Baláž P, Guilmeau E, Daneu N, Dobrozhan O, Baláž M, Hegedus M, Barbier T, Achimovičová M, Kaňuchová M, Briančin J. Tetrahedrites synthesized via scalable mechanochemical process and spark plasma sintering. Journal of the European Ceramic Society. 2020 May 1;40(5):1922-30.
Bella M, Rivero C, Blayac S, Basti H, Record MC, Boulet P. Oleylamine-assisted solvothermal synthesis of copper antimony sulfide nanocrystals: Morphology and phase control. Materials Research Bulletin. 2017 Jun 1;90:188-94.
Dutková E, Sayagués MJ, Fabián M, Baláž M, Achimovičová M. Mechanochemically synthesized ternary chalcogenide Cu3SbS4 powders in a laboratory and an industrial mill. Materials Letters. 2021 May 15;291:129566.
Fernandes PA, Shongalova A, da Cunha AF, Teixeira JP, Leitão JP, Cunha JM, Bose S, Salome PM, Correia MR. Phase selective growth of Cu12Sb4S13 and Cu3SbS4 thin films by chalcogenization of simultaneous sputtered metal precursors. Journal of Alloys and Compounds. 2019 Aug 15;797:1359-66.
Gur I, Fromer NA, Geier ML, Alivisatos AP. Air-stable all-inorganic nanocrystal solar cells processed from solution. Science. 2005 Oct 21;310(5747):462-5.
Jasieniak J, MacDonald BI, Watkins SE, Mulvaney P. Solution-processed sintered nanocrystal solar cells via layer-by-layer assembly. Nano letters. 2011 Jul 13;11(7):2856-64.
Jiasong Z, Weidong X, Huaidong J, Wen C, Lijun L, Xinyu Y, Xiaojuan L, Haitao L. A simple L-cystine-assisted solvothermal approach to Cu3SbS3 nanorods. Materials Letters. 2010 Jul 15;64(13):1499-502.
Kamat PV. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. The Journal of Physical Chemistry C. 2008 Dec 4;112(48):18737-53.
Kim SY, Kwak SG, Pi JH, Lee GE, Kim IH. Preparation of tetrahedrite Cu12Sb4S13 by mechanical alloying and hot pressing. Journal of Electronic Materials. 2019 Apr;48(4):1857-63.
Korostelev P.P. Gravimetric and titrimetric analysis in metallurgy. Handbook. Moscow, Metallurgy, 1985, 320 р.
Poudel B, Hao Q, Ma Y, Lan Y, Minnich A, Yu B, Yan X, Wang D, Muto A, Vashaee D, Chen X. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science. 2008 May 2;320(5876):634-8.
Ramasamy K, Sims H, Butler WH, Gupta A. Mono-, few-, and multiple layers of copper antimony sulfide (CuSbS2): a ternary layered sulfide. Journal of the American Chemical Society. 2014 Jan 29;136(4):1587-98.
Suehiro S, Horita K, Yuasa M, Tanaka T, Fujita K, Ishiwata Y, Shimanoe K, Kida T. Synthesis of copper–antimony-sulfide nanocrystals for solution-processed solar cells. Inorganic chemistry. 2015 Aug 17;54(16):7840-5.
Van Embden J, Tachibana Y. Synthesis and characterisation of famatinite copper antimony sulfide nanocrystals. Journal of Materials Chemistry. 2012;22(23):11466-9.
Van Embden J, Latham K, Duffy NW, Tachibana Y. Near-infrared absorbing Cu12Sb4S13 and Cu3SbS4 nanocrystals: synthesis, characterization, and photoelectrochemistry. Journal of the American Chemical Society. 2013 Aug 7;135(31):11562-71.
Wang MX, Yue GH, Fan XY, Yan PX. Properties and characterization of Cu3SbS3 nanowires synthesized by solvothermal route. Journal of crystal growth. 2008 Jun 1;310(12):3062-6.
Wang RY, Feser JP, Lee JS, Talapin DV, Segalman R, Majumdar A. Enhanced thermopower in PbSe nanocrystal quantum dot superlattices. Nano letters. 2008 Aug 13;8(8):2283-8.
Wuensch BJ. The crystal structure of tetrahedrite, Cu12Sb4S18. Zeitschrift für Kristallographie-Crystalline Materials. 1964 Nov 1;119(1-6):437-53.
Xu D, Shen S, Zhang Y, Gu H, Wang Q. Selective synthesis of ternary copper–antimony sulfide nanocrystals. Inorganic chemistry. 2013 Nov 18;52(22):12958-62.
Yang B, Wang L, Han J, Zhou Y, Song H, Chen S, Zhong J, Lv L, Niu D, Tang J. CuSbS2 as a promising earth-abundant photovoltaic absorber material: a combined theoretical and experimental study. Chemistry of Materials. 2014 May 27;26(10):3135-43.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

