PROPERTIES OF COMPOSITE WATER-COAL FUEL STABILIZED WITH CARBON SUBMICRON MATERIAL AND AMINO ALCOHOLS
DOI:
https://doi.org/10.15421/jchemtech.v32i2.299232Keywords:
Keywords: composite water-coal fuel, coal, amino alcohol, carbon, sedimentation, viscosity.Abstract
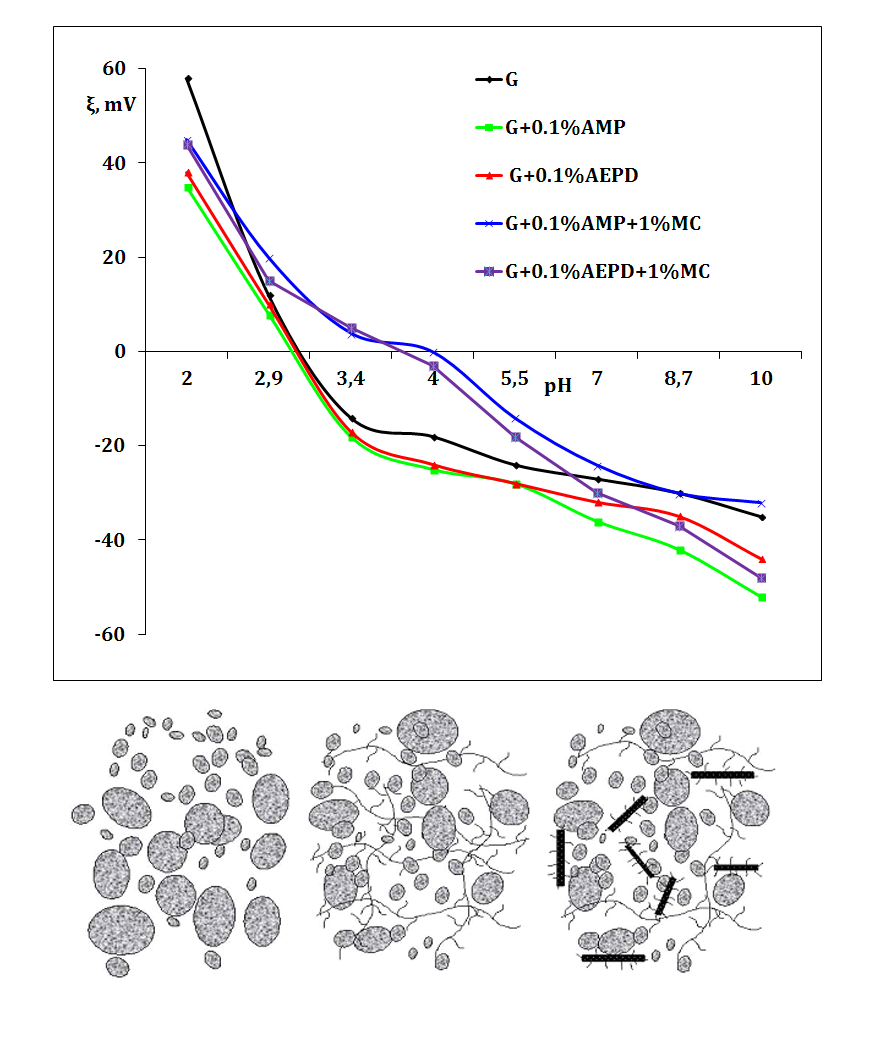
Composite Water-Coal Fuel (CWCF) is a viable alternative not only to solid coal but also to fuel oil and diesel fuel. The issue with combined systems is the low stability and heterogeneity of the distribution of coal particles, which causes an increase in the viscosity of dispersed systems. To regulate the rheological properties and stabilize the CWCF, additives such as dispersants, plasticizers, and stabilizers are used. The objective of the study was to examine the possibility of stabilizing composite water-coal fuel by adding amino alcohols and a submicron carbon substance (microdisperced carbon MC), obtained by plasma-chemical conversion of wastewater containing organics. The study focused on systems based on G-grade coal with a solid phase concentration of 61 %. Amino alcohols, 2-amino-2-methyl-1-propanol (AMP), and 2-amino-2-ethyl-1,3-propanediol (AEPD) were added to the CWCF at concentrations of 0.05 %, 0.1 %, 0.15 %, and 0.2 % by weight of CWCF. Its apparent viscosity was measured using a Rheotest-2 rheometer at a temperature of 20 °C, and the shear-stress/shear-rate data for the CWCF covered a range of 0.1 to 470 s-1. The sedimentation stability of the CWCF was assessed using a water-separating test and a storage period measured in days. The study found that the ξ-potential of G-grade coal particles in the presence of amino alcohols is 40–45 mV in absolute value. The obtained CWCFs demonstrate a pseudoplastic type of flow in the range of shear rates of 0-80 s-1. Systems with AMP are more stable than those with AERD. The introduction of highly dispersed carbon leads to an increase in the apparent viscosity of the systems and can be recommended for controlling the fluidity of the CWCF. To sum up, the sedimentation stability in the presence of highly dispersed carbon and amino alcohol additives increases from 5–6 to 10–14 days, almost twice. The sedimentation stability of these systems in the presence of МС makes the CWCFs suitable for short-term storage.
References
Jianzhong, L., Jianbin, W., Cong, C., Yongqiang, C., Xiangyang, Z. (2023). Preparing coal slurry from organic wastewater to achieve resource utilization: Slurrying performance and dispersant suitability. Fuel, 339, 126970. https://doi.org/10.1016/j.fuel.2022.126970
Dedi, L., Jianzhong, L., Shuangni, W., Jun, C. (2020). Study on coal water slurries prepared from coal chemical wastewater and their industrial application. Applied Energy, 268, 114976. https://doi.org/10.1016/j.apenergy.2020.114976
Enle, X., Zhenyong, M., Xiaofeng, J. (2023). Influence of waste brake oil on the rheological properties of coal-sludge water slurry. Environmental Science and Pollution Research, 14, 40886–40894. https://doi.org/10.1007/s11356-022-25040-y
Xiaofeng, J., Shixing, C., Lifeng, C., Enle, X., Hongji, C., Xianliang, M., Guoguang, W. (2022). Eco-friendly utilization of microplastics for preparing coal water slurry: rheological behavior and dispersion mechanism. Journal of Cleaner Production, 330, 129881. https://doi.org/10.1016/j.jclepro.2021.129881
Lin, L., Chuandong, M., Xiaoteng, L., Jianqiao, L., Hao, Y., Qingbiao, W., Zhenhua, W., Benlu, G., Xiaofang, Y. (2022). Study on the preparation of coal wastewater slurry from salt/alkali wastewater. Fuel, 315, 123612. https://doi.org/10.1016/j.fuel.2022.123612
Makarov, S., Klishchenko, R. E., Zavgorodnii, V. A., Makarova, E. V. (2011). The impact of the water salt content on the properties of coal-aqueous suspensions. J. Water Chem. Technol., 33(6), 601–611. https://doi.org/ 10.3103/s1063455x11060026
Liu, J.Z., Wang, S.N., Li, N., Wang, Y., Li, D.D., Cen, K.F. (2019). Effects of metal ions inorganic wastewater on coal water slurry and dispersant properties. Energy Fuels, 33, 7110. https://doi.org/10.1021/acs.energyfuels.9b01146
Makarov, A.S., Boruk, S.D., Egurnov, A.I., Dimitryuk, T.N., Klishchenko, R.E. (2014). Utilization of industrial wastewater in production of coal-water fuel. Journal of Water Chemistry and Technology, 36, 180. https://doi.org/10.3103/S1063455X14040055.
Lei, Z., Chen, W., Hengxiang, L., Wenjing, S., Xiaoliang, C., Yu, T., Junfeng, Z., Qian, M., Kang, Z. (2023). Design and evaluation of a novel dispersant with “surface-to-surface” adsorption function for preparing low-rank coal water slurry. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 667, 132357. https://doi.org/10.1016/j.colsurfa.2023.132357
Enle, X.., Shixing, C., Yaping, D., Zhenyong, M., Xiaofeng, J., Lifeng, C., Xianliang, M., Guoguang, W. (2021). The effect of isoamyl alcohol and sec-octyl alcohol on the viscosity of coal water slurry. Fuel, 292, 120394. https://doi.org/10.1016/j.fuel.2021.120394
Kang, Z., Yan, H., Zhi, Y., Tao, W., Xing, Z., Chen, W. (2022). Interactions of coal pitch with amphoteric polycarboxylate dispersant in coal pitch–water slurry: Experiments and simulations., Fuel, 318, 123608. https://doi.org/10.1016/j.fuel.2022.123608
Wenlin, S., Shiwei, W., Taotao, S., Hongfeng, Y., Yu, Z., Gang, Y., Zhonghua, L., Zhaokun, Q., Mei, Z. (2022). Improving the steric hindrance effect of linear sulfonated acetone–formaldehyde dispersant and its performance in coal–water slurry. RSC Advances, 55, 35508–35516. https://doi.org/10.1039/d2ra05802b
Zhang, WB., Luo, J., Huang, Y., Zhang, C., Du, L., Guo, J. (2020). Synthesis of a novel dispersant with topological structure by using humic acid as raw material and its application in coal water slurry preparation. Fuel, 262, 116576. https://doi.org/10.1016/j.fuel.2019.116576
Burnett, C. L., Bergfeld, W. F., Belsito, D. V., Klaassen, C. D., Marks, J. G., Shank, R. C., Slaga, T. J., Snyder, P. W., Andersen, А. F. (2009). Final Amended Report on Safety Assessment on Aminomethyl Propanol and Aminomethyl Propanediol. International Journal of Toxicology, 28(6), 141–161. https://doi.org/10.1177/1091581809350932
Wang, R.K., Ma, Q.Q., Zhao, Z., Ye, X.M., Jin, Q., Zhao, Z.H. (2019). Adsorption of surfactants on coal surfaces in the coking wastewater environment: kinetics and effects on the slurrying properties of coking wastewater-coal slurry. Ind. Eng. Chem. Res, 58, 12825. https://doi.org/10.1021/acs.iecr.9b01829
Testa, C., Zammataro, A., Pappalardo, A., Trusso, S. Giuseppe. (2019). Catalysis with carbon nanoparticles. RSC Advances, 9(47), 27659–27664. https://doi.org/ 10.1039/c9ra05689k
Zammataro, A., Sfrazzetto, G.T. (2019). Carbon Dots as Catalysts: A New Class of Nanozymes. Current Organocatalysis, 7(1), 2–6. https://doi.org/10.2174/2213337206666190702165008
Boehm H.P. (1994). Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon, 32(5), 759–769. https://doi.org/10.1016/0008-6223(94)90031-0
Alicia M. O., Sarah L. G., Katelyn R. H., Yasmin O. A., Heather А. А. (2010). Standardization of the Boehm titration: Part II. Method of agitation, effect of filtering and dilute titrant. Carbon, 48(4), 3313–3322. https://doi.org/10.1016/j.carbon.2009.11.050
Goncharuk, V. V.; Klishchenko, R. E.; Kornienko, I. V. (2017). Destruction of nonionic surfactants in a plasma-chemical reactor. Journal of Water Chemistry and Technology, 39(6), 641–649.
https://doi.org/ 10.3103/S1063455X1706008X
Goncharuk, V., Klishchenko, R., Kornienko, I. (2018). Destruction of GT Azo Active Orange Dye in the Flow–Through Plasma–Chemical Reactor. Journal of Water Chemistry and Technology, 40(4), 357–364. https://doi.org/ 10.3103/S1063455X1706008X
Maaß, S., Rojahn, J., Hänsch, R., Kraume, M. (2012). Automated drop detection using image analysis for online particle size monitoring in multiphase systems. Computers & Chemical Engineering, 45, 27–37. https://doi.org/10.1016/j.compchemeng.2012.05.014
Mishchuk, N., Kornilovich, B., Klishchenko, R. (2007), pH regulation as a method of intensification soil electroremediation. Colloids and Surfaces A: Physicochem. Eng. Aspects, 306(1-3), 171–179. https://doi.org/10.1016/j.colsurfa.2007.03.014
Duane G. L, Schlosberg R. H., B. G. Silbernagel. (1982). Understanding the Chemistry and Physics of Coal Structure (A Review), Proceedings of the National Academy of Sciences, 79(10), 57716. https://doi.org/10.2307/12417
Hamaker, H. C. (1938). London-V. D. Waals forces in colloidal systems. Recueil des Travaux Chimiques des Pays-Bas, 57(1), 62–72.
https://doi.org/ 10.1002/recl.19380570107
Qiang, L., Qian, W., Jian, H., Jiansheng, Z., Yang, Z. (2021). Aggregating structure in coal water slurry studied by e-DLVO theory and fractal dimension. Frontiers in Energy, 2, 306–316. https://doi.org/10.1007/s11708-021-0736-1
Usui, H., Saeki, T., Hayashi, K., Tamura, T. (1997). Sedimentation Stability and Rheology of Coal Water Slurries. Coal Preparation, 18(3-4), 201–214. https://doi.org/10.1080/07349349708905146
Raffi, M. T., Jamel. F, A., Dong-Jin, S., Lewis,. Wedgewood, E. (2002). Properties and rheology of coal–water mixtures using different coals. Fuel, 81(16), 2019–2033.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

