WATER PURIFICATION FROM NITRITE BY THE ENHANCED ULTRAFILTRATION METHOD
DOI:
https://doi.org/10.15421/jchemtech.v32i3.299283Keywords:
enhanced ultrafiltration, nitrite removal, membrane modification, polydopamine, membrane transportAbstract
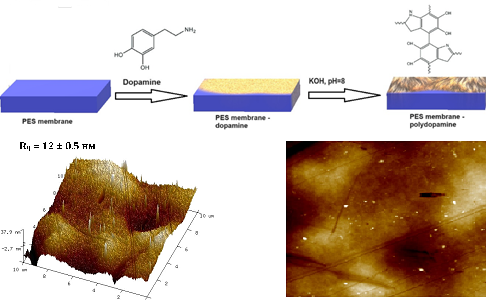
Commercial polyethersulfone membranes were modified to hydrophilize the surface and improve their transport properties for the nitrite removal process. The nitrites removal method from aqueous solutions is based on their ability to form complex due to binding with amino groups of the water-soluble polymer polyethyleneimine (PEI) in an acidic medium. The complex was separated using the modified membrane in the process of enhanced ultrafiltration. The surface of polyethersulfone membranes was modified using dopamine and polyacrylic acid in order to obtain a charged membrane surface, increase the water permeability coefficient, and improve transport properties. Modification conditions were established as the concentration of dopamine solution 10 mg/ml, pH = 8, T = 298 K, duration of modification 20 h, concentration of polyacrylic acid 0.5 wt.%, T = 298 K, duration of modification 2 h in the presence of EDAC. The effectiveness of the modification, the presence of new functional groups, and the change in surface morphology as a result of the modification were confirmed by infrared spectroscopy, scanning electron microscopy, and atomic force microscopy. The changes in the zeta potential of the membrane surface before and after modification were evaluated. The modification of the membranes leads to an increase in the mass transfer coefficient by 1.65 times and the water permeability coefficient by 1.2 times. The effect of pH, PEI concentration, and the presence of chlorides and sulfates on the process of nitrite extraction from solutions during ultrafiltration was studied. The optimal conditions for nitrite binding were determined as a pH value of 4; 0.5 wt.% concentration of PEI; and reaction time of 60 min. The ultrafiltration experiments of unmodified and modified PES020 commercial membranes were carried out. The productivity of modified membranes is higher than unmodified ones by 20–60 %, which compensates for a slight decrease in the retention coefficient in the process of nitrite removal. The retention coefficient of nitrites by this method is at least 70 %, which allows the effective use of this hybrid technology to remove nitrites from water in high concentrations.
References
Mendow, G., Grosso, C. I., Sánchez, A., Querini, C. A. (2017). Hybrid process for the purification of water contaminated with nitrites: Ion exchange plus catalytic reduction. Chem. Eng. Res. Des., 125, 348–360. https://doi.org/10.1016/j.cherd.2017.07.019
Xiang, X., Wang, J., Liu, Q., Peng, M., Zhao, Y., Li, Q., Tang, A.; Liu, Y., Liu, H.-B. (2021). Fabrication of PVDF/CdS/Bi2S3/Bi2MoO6 and Bacillus/PVA hybrid membrane for efficient removal of nitrite. Sep. Purif. Technol., 275, 119195. https://doi.org/10.1016/j.seppur.2021.119195
Ladychenko, V., Yara, O., Golovko, L., Serediuk, V. (2019). Groundwater Management in Ukraine and the EU. European Journal of Sustainable Development , 8, 1, 31-39 https://doi.org/10.14207/ejsd.2019.v8n1p31
Wadnerkar, P.D., Andrews, L., Wong, W.W., Chen, X., Correa, R.E., White, S., Cook, P.L., Sanders, C.J., Santos, I.R. (2021). Land use and episodic rainfall as drivers of nitrogen exports in subtropical rivers: Insights from δ15 N-NO3− , δ18 O-NO3− and 222Rn. Sci. Total. Environ., 758, 143669. https://doi.org/10.1016/j.scitotenv.2020.143669
US Environmental Protection Agency. National primary drinking water regulations, (2009). https://www.epa.gov/sites/default/files/2016-06/documents/npwdr_complete_table.pdf
World Health Organization, and WHO (2004), Guidelines for drinking-water quality. World Health Organization, Vol. 1. https://iris.who.int/handle/10665/42852
Feng L., Yang J., Yu H., Lan Z., Ye X., Yang G., Yang Q., Zhou J. (2020). Response of denitrifying community, denitrification genes and antibiotic resistance genes to oxytetracycline stress in polycaprolactone supported solid-phase denitrification reactor. Bioresour. Technol., 308, 123274. https://doi.org/10.1016/j.biortech.2020.123274
Li X., Li X., Li C., Li N., Zou P., Gao X., Cao Q. (2006). Nitrogen removal performances and metabolic mechanisms of denitrification systems using different volatile fatty acids as external carbon sources. Chem. Eng. J., 474, 145998. https://doi.org/10.1016/j.cej.2023.145998
Shen Z., Fang M., Tang L., Shi J., Wang W. (2024), Pd/Cu bimetallic nano-catalyst supported on anion exchange resin (A520E) for nitrate removal from water: High property and stability. Environ. Res., 241, 117616 https://doi.org/10.1016/j.envres.2023.117616
Epsztein R., Nir O., Lahav O., Green M. (2015), Selective nitrate removal from groundwater using a hybrid nanofiltration–reverse osmosis filtration scheme. Chem. Eng. J, 279, 372–378. https://doi.org/10.1016/j.cej.2015.05.010Get rights and content
Popova A., Rattanakom R., Yu Z.-Q., Li Z., Nakagawa K., Fujioka T. (2023), Evaluating the potential of nanofiltration membranes for removing ammonium, nitrate, and nitrite in drinking water sources. Water Res. 244, 120484. https://doi.org/10.1016/j.watres.2023.120484
Shi, Y., Wang, J., Wan H., Wan, D., Wang, Y., Li, Y. (2023), Effective removal of nitrate in water by continuous-flow electro-dialysis ion exchange membrane bioreactor (CF-EDIMB): Performance optimization and microbial analysis. Chemosphere, 341, 139880. https://doi.org/10.1016/j.chemosphere.2023.139880
Tabatabai, A., Scamehorn, J.F., Christian, S.D. (1995), Economic feasibility study of polyelectrolyte-enhanced ultrafiltration (PEUF) for water softening. J. Membr. Sci., 100, 193–207. https://doi.org/10.1016/0376-7388(94)00220-S
Rodriguez Pastof M.; Samper-Vidalb E.; Varo Galvhnb P.; Prats Ricoa D. (2003), Analysis of the variation in the permeate flux and of the efficiency of the recovery of mercury by polyelectrolyte enhanced ultrafiltration (PE-UF). Desalination, 151, 247–251. https://doi.org/10.1016/S0011-9164(02)01017-2
Zhua X., Choob K.-H.; Park J.-M. (2021), Nitrate removal from contaminated water using polyelectrolyte-enhanced ultrafiltration. Desalination., 193, 350–360 https://doi.org/10.1016/j.desal.2005.06.067
Santoro, S., Timpano, P., Avci, A. H., Argurio, P., Chidichimo, F., De Biase, M., Straface, S., Curcio, E. (2021). An integrated membrane distillation, photocatalysis and polyelectrolyte-enhanced ultrafiltration process for arsenic remediation at point-of-use. Desalination., 520, 115378. https://doi.org/10.1016/j.desal.2021.115378
Abdallah, S., Abu-Khader, M. M., Badran, O. (2009), Effect of various absorbing materials on the thermal performance of solar stills. Desalination., 242, 128–137. https://doi.org/10.1016/j.desal.2008.03.036
Mondal, S., Ben Mlouka, S., Dhahbi, M., De, S. (2011), A physico-chemical model for polyelectrolyte enhanced ultrafiltration. J. Membr. Sci., 376, 142–152. https://doi.org/10.1016/j.memsci.2011.04.011
Bodzek, M., Korus, I., Loska, K. (1999), Application of the hybrid complexation - ultrafiltration process for removal of metal ions from galvanic wastewater. Desalination., 121, 117–121. https://doi.org/10.1016/S0011-9164(99)00012-0
Roa, K., Boulett, A., Sanchez, J. (2023). Removal of Cr(VI) by ultrafiltration enhanced by a cellulose-based soluble polymer. Journal of Water Process Engineering. , 103478. https://doi.org/10.1016/j.jwpe.2022.103478
Oyarce, E., Butter, B., Sánchez, J. (2023). Polyelectrolytes applied to remove methylene blue and methyl orange dyes from water via polymer-enhanced ultrafiltration. Journal of Environmental Chemical Engineering. 106297. https://doi.org/10.1016/j.jece.2021.106297
Bubela, H., Konovalova, V., Kujawa, J., Kolesnyk, I., Burban, A., Kujawski, W. (2023). Enhancing of transport parameters and antifouling properties of PVDF membranes modified with Fe3O4 nanoparticles in the process of proteins fractionation, Sep. Purif. Technol., 325, 124573. https://doi.org/10.1016/j.seppur.2023.124573
Chang, Z., Ting, L., Jing, L., Sum, Ya. (2023). Insight into polydopamine coating on microfiltration membrane with controlled surface pore size for enhanced membrane rejection Polymer, 287, 126446. https://doi.org/10.1016/j.polymer.2023.126446
Konovalova, V., Kolesnyk, I., Burban A.; Kujawski, W., Knozowska, K., Kujawa, J. (2019). Improvement of separation and transport performance of ultrafiltration membranes by magnetically active nanolayer. Colloids Surf. A: Physicochem. Eng. Asp., 569, 67–77. https://doi.org/10.1016/j.colsurfa.2019.02.061
Okyere Abayie, S., Leiviskä, T. (2022). Removal of nitrate from underground mine waters using selective ion exchange resins, Journal of Environmental Chemical Engineeringm, 10, 108642 https://doi.org/10.1016/j.jece.2022.108642
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

