THE DEGRADATION OF D-GLUCOSE IN ACIDIC AQUEOUS SOLUTION
DOI:
https://doi.org/10.15421/jchemtech.v32i3.299431Keywords:
glucose; glucose degradation products; 3,4-di-deoxyglucosone-3-ene; 5-hydroxymethylfurfural; acidic compounds.Abstract
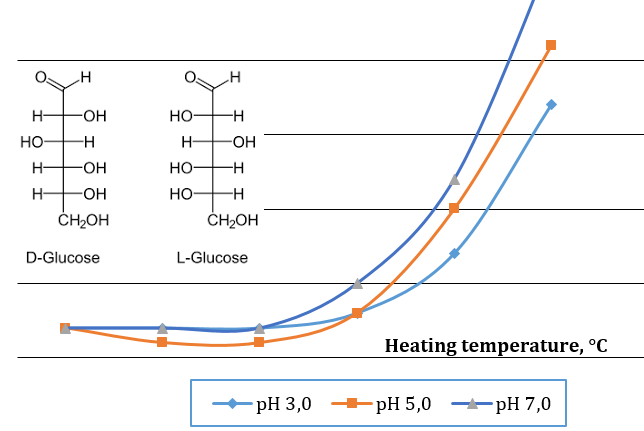
The objective of this work was to estimate glucose degradation products (GDPs) in model system D-glucose/H+ based on the changes in the pH values, absorption of ultraviolet (UV) light and integral intensities of carbonyl absorption (ν 1740–1710 cm-1) by Raman spectroscopy. Spectrophotometric, pH-metric and Raman spectroscopy methods were used to elucidate mechanism of both glucose degradation and transformation of GDPs in the model D-glucose solutions after desired time and temperature of heating. Common features for all the tested solutions after heating were reduce in pH values and increase in UV absorbance at λmax 225 nm (absorbance of 3,4-DGE) and in the carbonyl region λmax 280-285 nm (absorbance of 5-HMF). This indicated that these intermediates had been formed during heating. Their stability increases with a decrease in initial pH values. By Raman and UV spectroscopy methods temperature zones were determined in which certain processes of transformation of D-glucose were seen. It was demonstrated that in the temperature interval at 80–95 °C at all pH values, formation of 3,4-DGE accelerated while the their transformation into 5-HMF slowed down. It was shown that the formation of unsaturated carbonyl-containing compounds (3,4-DGE), as starting intermediates for further transformations were slowed down at pH 3, which indicates a certain stability of D-glucose under these conditions.
References
Aktağ, I. G., Gökmen, V. (2021). Investigations on the formation of α-dicarbonyl compounds and 5-hydroxymethylfurfural in apple juice, orange juice and peach puree under industrial processing conditions. European Food Research and Technology, 247(4), 797–805. https://doi.org/10.1007/s00217-020-03663-0.
Jost, T., Henning, C., Heymann, T., & Glomb, M. A. (2021). Comprehensive analyses of carbohydrates, 1,2-dicarbonyl compounds, and advanced glycation end products in industrial bread making. Journal of Agricultural and Food Chemistry, 69(12), 3720–3731. https://doi.org/10.1021/acs.jafc.0c07614.
Maasen, K., Scheijen, J. L. J. M., Opperhuizen, A., Stehouwer, C. D. A., Van Greevenbroek, M. M., Schalkwijk, C. G. (2021). Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chemistry, 339, 128063. https://doi.org/10.1016/j.foodchem.2020.128063.
Zhang, J., Wei, F., Zhang, T., Cui, M., Peng, B., Zhang, Y., Wang, S. (2022). Simultaneous determination of seven α-dicarbonyl compounds in milk and milk products based on an LC-MS/MS method with matrix-matched calibration. Food Analytical Methods, 15(6), 1652–1662. https://doi.org/10.1007/s12161-021-02219-6.
Gensberger-Reigl, S., Weigel, I., Stützer, J., Auditore, A., NikolausT., Pischetsrieder M. (2022). Degradation and de novo formation of nine major glucose degradation products during storage of peritoneal dialysis fluids. Sci Rep., 12, 4268. https://doi.org/ 10.1038/ s41598-022-08123-1.
Leitzen, S., Vogel, M., Steffens, M., Zapf, T., Müller, C. E., Brandl, M. (2021). Quantification of Degradation Products Formed during Heat Sterilization of Glucose Solutions by LC-MS/MS: Impact of Autoclaving Temperature and Duration on Degradation. Pharmaceuticals, 14(11), 1121. https://doi.org/10.3390/ph14111121.
Gökmen, V. (2020). A survey of the occurrence of α-dicarbonyl compounds and 5-hydroxymethylfurfural in dried fruits, fruit juices, puree and concentrates. Journal of Food Composition and Analysis. 91, 103523. https://doi.org/10.1016/ mj.jfca.2020.103523.
Aktağ, I.G., Gökmen, V. (2020). Multiresponse kinetic modelling of α-dicarbonyl compounds formation in fruit juices during. Food Chemistry. 320, 126620. https://doi.org/10.1016/j.foodchem.2020.126620.
de Souza, R.L., Yu, H., Rataboul, F., Essayem, N. (2012). 5-Hydroxymethylfurfural (5-HMF) Production from Hexoses: Limits of Heterogeneous Catalysis in Hydrothermal Conditions and Potential of Concentrated Aqueous Organic Acids as Reactive Solvent System. Challenges, 3(2), 212–232. https://doi.org/10.3390/challe3020212.
Jianmei, L., Xiaorong, L., Zhongzheng, C. (2022). [Effect of Reaction Conditions on the Formation of 3-Deoxyglucosone and 5-Hydroxymethylfurfural in Sugar-acid Reaction System]. J. Science and Technology of Food Industry, 43(2), 93−100 (in Chinese). https://doi.org/10.13386/j.issn1002-0306.2021040327.
Liang, J., Jiang, J., Cai, T., Liu, C., Ye, J., Zeng, X., Wang, K. (2023). Advances in selective conversion of carbohydrates into 5-hydroxymethylfurfural. Green Energy & Environment, 9(9), 1384-1406
, https://doi.org/10.1016/j.gee.2023.11.005.
Jadhav, H., Pedersen, C. M., Sølling, T. I, Bols, M. (2011). 3-Deoxy-glucosone is an Intermediate in the Formation of Furfurals from D-Glucose. ChemSusChem., 4(8), 1049–51. https://doi.org/10.1002/cssc.201100249
Huang, H., Chen, J., Zheng, M., Zhang, L., Ji, H., Cao, H., Dai, F., Wang, L. (2023). Precursors and formation pathways of furfural in sugarcane juice during thermal treatment. Food Chemistry, 402, 134318. https://doi.org/10.1016/j.foodchem.2022.134318.
Herraiz, T., Vera, F. (2021). Occurrence, Formation from D-Fructose and 3-Deoxyglucosone, and Activity of the Carbohydrate-Derived β-Carbolines in Foods. Agric. Food Chem., 69(23), 6650–6664. https://doi.org/10.1021/acs.jafc.1c02281.
Martins, F.C.O.L., Alcantara, G.M.R.N., Silva, A.F.S., Melchert, W.R., Rocha, F.R.P. (2022). The role of 5-hydroxymethylfurfural in food and recent advances in analytical methods. Food Chemistry. 395, 133539. https://doi.org/10.1016/j.foodchem.2022.133539.
Zhou, X., Zhang, Z., Liu, X., Wu, D., Ding, Y., Li, G., & Wu, Y. (2020). Typical reactive carbonyl compounds in food products: Formation, influence on food quality, and detection methods. Comprehensive Reviews in Food Science and Food Safety, 19(2), 503–529. https://doi.org/10.1111/1541-4337.12535.
Yan, S., Wu, L., Xue, X. (2023). α-Dicarbonyl compounds in food products: Comprehensively understanding their occurrence, analysis, and control. Comprehensive Reviews in Food Science and Food Safety, 22(2), 1387–1417. https://doi.org/ 10.1111/1541-4337.13115.
Cincotta, F., Brighina, S., Condurso, C., Arena, E., Antonella, V., Fallico, B. (2021). Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing. Foods, 10(9), 2101. https://doi.org/ 10.3390/foods10092101.
Huang, X., Duan, H., Barringer, S. A. (2011). Effects of buffer and temperature on formation of furan, acetic acid and formic acid from carbohydrate model systems. LWT - Food Science and Technology, 44, 1761–1765. https://doi.org/10.1016/j.lwt.2011.03.016
Körner, P., Jung, D., Kruse, A. (2019). Influence of the pH Value on the Hydrothermal Degradation of Fructose. Chemistry Open, 8(8), 1121–1132. https://doi.org/10.1002/open.201900225.
Chiku, A., Ohfuji, K., Ohtake, N., Yoshida, M., Ono, H., Kitaoka, M. (2022). Isomerization of 6-O-substituted glucose and fructose under neutral pH conditions and subsequent β-elimination reactions. Carbohydrate Research, 519, 108626. https://doi.org/10.1016/ j.carres. 2022.108626.
Chen, K., Prabel, J., Dutton, J., Gotoda, M., Asai, Y., Grobin, A. (2015). Trans-3,4-dideoxyglucone-3-ene (trans-3,4-DGE), a most reactive glucose degradation product in freshly heat sterilized glucose solutions. Carbohydrate Research. 418, 57–64. https://doi.org 10.1016/j.carres.2015.10.003.
Qian, X., Nimlos, M. R., Himmel, M. E. (2005). Ab initio molecular dynamics simulations of β-D-glucose and β-D-xylose degradation mechanisms in acidic aqueous solution. Carbohydrate Research., 340(14), 2319–2327. https://doi.org/10.1016/j.carres. 2005.07.021.
Alkarkhi, A. F.M., Alqaraghuli, W.A.A., Yusup, Y., Abu Amr, S. S., Mahmud, M.N., Dewayantoa, N. (2019). Data on the absorbance of glucose during the acid hydrolysis of the sugarcane bagasse. Data in Brief, 24, 103894. https://doi.org/10.1016/j.dib. 2019.103894.
Bi, Y.-X., Zielinska, S., Ni, J.-B., Li, X.-X., Xue, X.-F., Tian, W.-L., Peng, W.-J., Fang, X.-M. (2022). Effects of hot-air drying temperature on drying characteristics and color deterioration of rape bee pollen. Food Chemistry: X. 16, 100464. https://doi.org/10.1016/j.fochx. 2022.100464.
Woo, K.S., Kim, H.Y., Hwang, I.G., Lee, S.H., Jeong, H.S. (2015). Characteristics of the Thermal Degradation of Glucose and Maltose Solutions. Prev. Nutr. Food Sci. 20(2), 102–109. https://doi.org/10.3746/pnf.2015.20.2.102.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

