PHASE EQUILIBRIA IN THE La2O3-Lu2O3-Ho2O3 SYSTEM AT 1500 °С
DOI:
https://doi.org/10.15421/jchemtech.v32i3.303869Keywords:
phase equilibria, lanthana, lutetia, holmia, isothermal section, solid solutions, lattice parametersAbstract
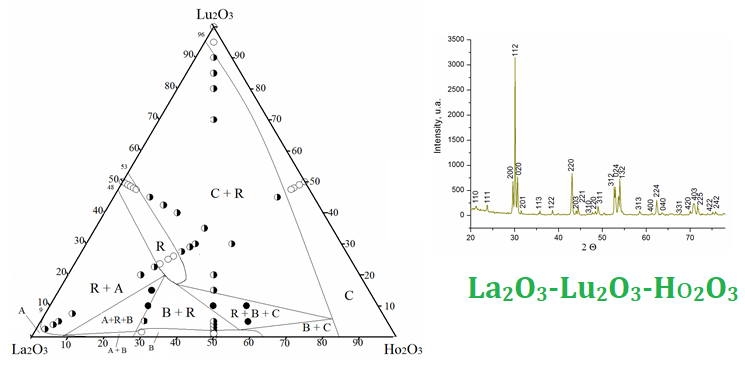
The phase equilibria in the La2O3–Lu2O3–Ho2O3 ternary system at 1500 °C were studied by X-ray diffraction (XRD) and scanning electron microscopy (SEM) in the whole concentration range. La2О3, Lu2О3, and Ho2О3 (99.99 %) were used as starting substances. The experimental samples were prepared with a concentration step of 1–5 mol%. The oxides were dissolved in HNO3 (1 : 1) followed by evaporation of the solutions and decomposition of nitrates at 800 °C for 2 hours. The samples were heat treated at 1500 °C (for 70 h) in air. The phase composition of the test samples studied by X-ray diffraction (XRD, DRON-3), microstructural phase and electron microprobe X–ray (Superprobe-733, JEOL, Japan, Palo Alto, CA) analyses. Solid solutions based on various polymorphic forms of original oxides and ordered LaLuO3 phase were detected in the system. No new phases were found in the system. The isothermal cross-sections of the La2O3–Lu2O3–Ho2O3 phase diagram at 1500 °C are characterized by the presence of four single-phase (A-La2O3, B-La2O3, R, C-Lu2O3 (Ho2O3)), five two-phase (C + R, A + R, B + A, B + R, B + C) and two three-phase ( A + R+ B, B + R + C) regions. Solubility limits are determined and concentration dependences of periods also lattice parameters of the unit cell of phases formed in the system are constructed. The range of homogeneity of solid solutions based on the R-phase extends from 0 to 8 mol% Ho2O3 and from ~47 to 54 mol% La2O3 at 1500 °C. Lu and Ho oxides form an continuous series of C-REE oxide solid solutions.
References
Milisavljevic, I., Zhang, M., Jiang, Q., Liu, Q., Wu, Y. (2025). Transparent Electro-Optic Ceramics: processing, materials, and applications, Journal of Materiomics, 11(2), 100872. https://doi.org/10.1016/j.jmat.2024.04.002
Peng, L., Yang, J., Han, T., Lang, T., Cao, S., Liu, B., Qiang, Q., Chen, W. (2023). Tunable emission and high chromogenic laser lighting of transparent ceramics for high-brightness white LEDs/LDs, Journal of Luminescence, 263, 119987, https://doi.org/10.1016/j.jlumin.2023.119987
Mo, J., Zhang, L., Hu, C., Wang, Y., Chen, H., Li, X., Wu, J., Cheng, Z., Li, T., Li, D. J. (2024). Fabrication of submicron grained alumina transparent ceramics with high bending strength and low dielectric loss, Ceramics International, 50(16), 28301-28308. https://doi.org/10.1016/j.ceramint.2024.05.131
Jing, Y., Tian, F., Guo L., Li, T., Junlin Wu, J., Ivanov, M., Hreniak, D., Li, J. (2024). Effect of TEOS content on microstructure evolution and optical properties of Sm:YAG transparent ceramics, Optical Materials, 147, 114681. https://doi.org/10.1016/j.optmat.2023.114681
Hu, D., Zhang, L., Tian, F., Zhu, D., Chen, P., Yuan, Q., Balabanov, S., Li, J. (2023). Fine-grained transparent Dy2O3 ceramics fabricated from precipitated powders without sintering aids, Optical Materials, 142, 114071. https://doi.org/10.1016/j.optmat.2023.114071
Akinribide, O.J., Mekgwe, G. N., Akinwamide, S. O., Gamaoun F., Abeykoon, C., Johnson, O. T., Olubambi, P. A. (2022). A review on optical properties and application of transparent ceramics, J. Mater. Res. Technol., 21, 712–738. https://doi.org/10.1016/j.jmrt.2022.09.027
Wan, Z., Li, W., Bei, M., Liu, Z., Yang, Yu. (2020). Fabrication and spectral properties of Ho-doped calcium fluoride transparent ceramics, Journal of Luminescence, 223, 117188. https://doi.org/10.1016/j.jlumin.2020.117188
Ye, Y., Tang, Z., Ji, Z., Xiao, H., Liu, Y., Qin, Y., Liang, L., Qi, J., Lu, T. (2021). Fabrication and luminescent properties of holmium doped Y2Zr2O7 transparent ceramics as new type laser material, Optical Materials, 121, 111643. https://doi.org/10.1016/j.optmat.2021.111643
Ajmala, M., Alib, T., Adil Khana, M., Ahmada, S., Ahmad Mianb, S., Waheeda, A., Ali, S. (2017). Structural and optical properties of La2O3:Ho3+ and La(OH)3 : Ho3+ crystalline particles, Materials Today: Proceedings, 4 4900–4905. doi.org/10.1016/j.matpr.2017.04.093
Yang, Q., Zhou, H., Xu, J., Su, L. (2008). Synthesis and luminescence characterization of cerium doped Lu2O3-Y2O3-La2O3 solid solution transparent ceramics, Optics express., 16, 12295. doi:10.1364/oe.16.012290.
Le, T. H., Phan, A.-L., Ty, N.M., Zhou, D., Qiu, J., Dan. H.K. (2021). Influences of copper–potassium ion exchange process on the optical bandgaps and spectroscopic properties of Cr3+/Yb3+ co-doped in lanthanum aluminosilicate glasses, RSC Advances, 11(15), 8917–8926. https://doi.org/10.1039/d0ra10831f
Muller-Buschbaum, Hk., Graebner, P. H. (1971). [Zur Kristallstruktur von LaErO3 und LaLuO3], Z. Anorg. Allg. Chem., 386, 158–162 (in German).
Xiong, K., Robertson, J. (2009). Electronic structure of oxygen vacancies in La2O3, Lu2O3 and LaLuO3, Microelectr. En., 86(7–9), 1672–1675. https://doi.org/10.1016/j.mee.2009.03.016
Chudinovych, O. V., Zhdanyuk, N.V. (2020). [Interaction of lanthanum oxides and lutetium at a temperature of 1500–1600 °С], Ukrainian Chem. J., 86 (30), 19–25 (in Ukrainian). https://doi.org/10.33609/0041-6045.86.3.2020.19-25
Zinkevich, M. (2007). Thermodynamics of rare earth sesquioxides, Prog. Mater. Sci., 52(4), 597–647. https://doi.org/10.1016/j.pmatsci.2006.09.002.
Coutures, J.P., Foex, M. (1976). Etude a Haute TempCrature des Systsmes Formes par le Sesquioxyde de Lanthane et les Sesquioxydes de Lanthanides. I. Diagrammes de Phases (1400 ◦C https://doi.org/10.1016/0022-4596(76)90218-8.
Coutures, J., Sibieude, F., Foex, M. (1976). Etude a haute temp´erature des syst`emes form´espar les sesquioxydes de lanthane avec les sesquioxydes de lanthanides. II. Influence de la trempe sur la nature des phases obtenues `a la temp´erature ambiante, J. Solid State Chem., 17, 377–384, https://doi.org/10.1016/S0022-4596(76)80006-0.
Berndt, V., Maier, D., Keller, C. (1975). New ABO3 interlanthanide perovskite compounds, J. Solid State Chem., 13(1–2), 131–135. https://doi.org/10.1016/0022-4596(75)90090-0.
Korniienko, O.A., Yushkevich, S.V., Bykov, О. І., Sameliuk, A.V., Bataiev, Yu. M., Zamula, M.V. (2023). Phase relation studies in the CeO2-La2O3-Ho2O3 system at temperature of 1500 °С, Mater. Today Commun., 35, 105789. https://doi.org/10.1016/j.mtcomm.2023.105789
Zhang, Y. (2016). Thermodynamic Properties of Rare Earth Sesquioxides, McGill University, Montreal, QC, Canada.
Andrievskaya, E.R. (2010). [Phase Equilibria in the Systems of Hafnia, Yttria with Rare-Earth Oxides.] Scientific Book Project, Naukova Dumka, Kiev, 2010. (in Russian).
Zinkevich, M. Thermodynamic Database for Rare Earth Sesquioxide. https://materialsdata.nist.gov/handle/11256/965.
Chudinovych, O. V., Andrievskaya, О. R., Bogatyryova, J. D., Kovylyaev, V. V., Bykov, O. I. (2021). Phase equilibria in the La2O3–Y2O3–Nd2O3 system at 1500 °С, J. Eur. Ceram. Soc., 41, 6606–6616. https://doi.org/10.1016/j.jeurceramsoc.2021.06.017
Chudinovych, O. V., Bykov, О. І., Sameliuk, A.V. (2022). Phase equilibria in the La2O3–Y2O3–Gd2O3 system at 1500 °C, Process. Appl. Ceram., 16(4), 328–334. doi.org/10.2298/PAC2204328C
Chudinovych, O. V., Bykov, О. І., Sameliuk, A.V. (2021). Interaction of lanthanum, lutetium, and ytterbium oxides at 1600 °C, Powder Metallurgy and Metal Ceramics., 60(5-6), 337–346. https://doi.org/10.1007/s11106-021-00248-8
Chudinovych, O. V., Bykov, О. І., Sameliuk, A.V. (2021). Phase relation studies in the La2O3–Lu2O3–Yb2O3 system at 1500 °С, Journal of Chemistry and Technologies, 29(4), 485–494. doi.org/10.15421/jchemtech.v29i4.238943
Chudinovych, O. V., Shyrokov, O. V., Sameliuk, A.V. (2023). Phase equilibria in the La2O3–Lu2O3–Er2O3 system at 1500 and 1600 °С, Journal of chemistry and technologies, 31(1), 51–60. doi:10.15421/jchemtech.v31i1.27149
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

