NATURAL ALUMINOSILICATES MODIFIED WITH ARENESULFONIC ACID FRAGMENTS AS CATALYSTS FOR ACETALIZATION OF GLYCEROL WITH CYCLOHEXANONE
DOI:
https://doi.org/10.15421/jchemtech.v32i3.304786Keywords:
catalyst, reaction rate, kinetics, activity, alumosilicates, glycerol, cyclohexanone, ketalAbstract
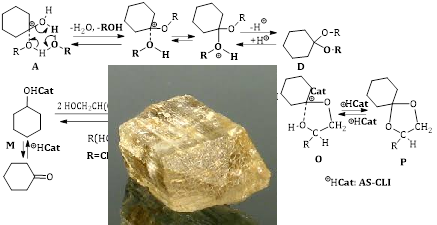
The kinetics of the reaction of glycerol with cyclohexanone in the presence of natural aluminosilicates of the domestic deposit (bentonite, clinoptilolite and trepel) modified with fragments of arenesulfonic acid were studied. The influence of the mass fraction of the catalyst, the reaction temperature and the molar ratio of the reagents on glycerol conversion was determined. It was found that in the presence of bentonite and trepel, unlike clinoptilolite, regardless of the influence of these factors, the glycerol conversion reaches ~100 %. The main product of the reaction is the five-membered cyclic ketal 1.4-dioxaspiro[4,5]decane-2-methanol with a selectivity of 99 % (determined on gas chromatograph Shimadzu GC-2030, Japan). The adsorption and acidic characteristics of the catalysts (pore size, surface area, Brønsted and Lewis acid centers) were studied and the influence of these characteristics on their catalytic properties was compared. It was established that the activity of the catalyst is due to a larger surface area and pore structure. Using the method of «double inverse values» (Lineweaver-Burk plot method), the features of the reaction were investigated, the Michaelis constants and maximum reaction rates and the concentration order of glycerol were determined. A possible reaction mechanism under conditions of heterogeneous acid catalysis is proposed. It is shown that the formation of the cyclohexanone-catalyst complex at the same rate is common to the three samples of catalysts and the subsequent «route» of the reaction differs depending on the concentration order of glycerol.
References
Tabatabaei, M., Aghbashlo, M., Dehhaghi, M., Kazemi Shariat Panahi, H., Mollahosseini, A., Hosseini, M., Mojarab Soufiyan, M. (2019). Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci., 74, 239–303. https://doi.org/10.1016/j.pecs.2019.06.001
Ramos, M., Soares Dias, A. P., Puna, J. F., Gomes, J., Bordado, J. C. (2019). Biodiesel production processes and sustainable raw materials. Energies, 12, 4408–4438 https://doi.org/10.3390/en12234408
Almeida, E. L., Olivo, J. E., Andrade, C. M. G. (2023). Production of biofuels from glycerol from the biodiesel production process – A brief review. Fermentation, 9, 869–887. https://doi.org/10.3390/fermentation9100869
Liu, Y., Zhong, B., Lawa, A. (2022). Recovery and utilization of crude glycerol, a biodiesel byproduct. RSC Adv., 12(43), 27997–28008. https://doi.org/10.1039/D2RA05090K
Nomanbhay, S., Ong, M. Y., Chew, K. W., Show, P.-L., Lam, M. K., Chen, W.-H. (2020). Organic carbonate production utilizing crude glycerol derived as by-product of biodiesel production: A review. Energies, 13, 1483–1505. https://doi.org/10.3390/en13061483
Ao, S., Rokhum, S. L. (2022). Recent advances in the valorization of biodiesel by-product glycerol to solketal. J. Chem., 1, 1–18. https://doi.org/10.1155/2022/4938672
Trifoi, A. R., Agachi, P. S., Pap. T. (2016). Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renewable Sustainable Energy Rev., 62, 804–814. https://doi.org/10.1016/j.rser.2016.05.013
Nanda, M. R., Yuan, Z., Qin, W., Ghaziaskar, H. R., Poirier, M.-A., Xu, C. C. (2014). Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel, 117, 470–477. https://doi.org/10.1016/j.fuel.2013.09.066
Garcıa, E., Laca, M., Pe´rez, E., Garrido А., Peinado, J. (2008). New class of acetal derived from glycerin as a biodiesel fuel component. Energy Fuels, 22(6), 4271–4280. https://doi.org/10.1021/ef800477m
Mota, C. J. A., da Silva, C. X. A., Rosenbach, Jr. N., Costa, J., da Silva F. (2010). Glycerin derivatives as fueladditives: the addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels, 24(4), 2733–2736. https://doi.org/10.1021/ef9015735
Olson, A. L., Tun´er, M., Verhelst, S. (2023). A concise review of glycerol derivatives for use as fuel additives. Heliyon, 9, 13041–13054. https://doi.org/10.1016/j.heliyon.2023.e13041
Raman, A. A. A., Tan, H. W., Buthiyappan, A. (2019). Two-step purification of glycerol as a value added by product from the biodiesel production process. Front. Chem., 7, 774-782. https://doi.org/10.3389/fchem.2019.00774
Attarbachi, T., Kingsley, M. D., Spallina, V. (2023). New trends on crude glycerol purification: A review. Fuel, 340, 127485. https://doi.org/10.1016/j.fuel.2023.127485
Davtian, A. S., Levchenko, O. O., Chikhichin, D. G., Yaremov, P. S., Kamalov, G. L. (2021). [Acetalization of glycerine at presence of acid-modified clinoptilolite, bentonite and tripolite from the domestic deposits]. New functional substances and materials for hemical engineering. «Akademperiodyka», 296–310 (in Ukrainian). https://doi.org/10.15407/akademperiodyka.444.296
Davtian, A. S., Chikhichin, D. G., Levchenko, O. O., Kamalov, G. L. (2024). Effect of the acidic treatment on the catalitic properties of natural aluminosilicates in glycerol acetylation. Theor. Exp. Chem., 59(6), 412–417.
Davtian, A. S., Chikhichin, D. G., Levchenko, O. O., Kamalov, G. L. (2023). [Interaction of glycerol with acetone in the presence of acid-modified clinoptilolite]. Visn. Odes. nac. univ., Him., 28(3(86)), 28–34 (in Ukrainian). https://doi.org/10.18524/2304–0947.2023.3(86).297809
Tangestanifard, M., Ghaziaskar, H. S. (2017). Arenesulfonic acid-functionalized bentonite as catalyst in glycerol esterification with acetic acid. Catalysts, 7(7), 211–221. https://doi.org/10.3390/catal7070211
Emeis, C. A. (1993). Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal., 141(2), 347–354. http://dx.doi.org/10.1006/jcat.1993.1145
Alothamn, Z. A. (2012). A Review: Fundamental aspects of silicate mesoporous materials. Mater., 5(12), 2874–2902. https://doi.org/10.3390/ma5122874
Guzenko, N. V., Lodewyckx, P., László, K., Thommes, M. (2019). [The features of water vapour adsorption on micro- and mesoporous activated carbons]. Ximiya, fizyka ta texnologiya poverxni, 10(1), 22–37 (in Ukrainian). https://doi.org/10.15407/hftp10.01.022
Bardestania, R., Patienceb, G. S., Kaliaguine, S. (2019). Experimental methods in chemical engineering: specific surface area and pore size distribution measurements – BET, BJH, DFT. The Canadian Journal of Chemical Engineering, 97(11), 2781–2791. https://doi.org/10.1002/cjce.23632
L´opez-P´erez, L., Zarubina, V., Meli´an-Cabrera, I. (2021). The Brunauer–Emmett–Teller model on alumino-silicate mesoporous materials. How far is it from the true surface area? Microporous Mesoporous Mater., 319, 111065. https://doi.org/10.1016/j.micromeso.2021.111065
Zholobenko, V., Freitas, C., Jendrlin, M., Bazin, P., Travert, A., Thibault-Starzyk, F. (2020). Probing the acid sites of zeolites with pyridine: quantitative AGIR measurements of the molar absorption coefficients. J. Catal., 385, 52–60. https://doi.org/10.1016/j.jcat.2020.03.003
Vifttaria, M., Nurhayati, Anita, S. (2019). Surface acidity of sulfuric acid activated Maredan clay catalysts with boehm titration method and pyridine adsorption-FTIR. J. Phys.: Conf. Ser., 1351(1) 012040. doi:10.1088/1742-6596/1351/1/012040
Sun, S., He, M., Dai, Y., Li, X., Liu, Z., Yao, L. (2017). Catalytic acetalization: An efficient strategy for high-value utilization of biodiesel-derived glycerol. Catalysts, 7(6), 184–194. https://doi.org/10.3390/catal7060184
Amri, S., Gómez, J., Balea, A., Merayo, N., Srasra, E., Besbes, N., Ladero, M. (2019). Green production of glycerol ketals with a clay-based heterogeneous acid catalyst. Appl. Sci., 9(21), 448–-4509. https://doi.org/10.3390/app9214488
Segel, I. H. (2013). Enzyme kinetics. Encycl. Biol. Chem. (2nd Ed.), 216–220. https://doi.org/10.1016/B978-0-12-378630-2.00012-8
Yadav, G. D., Yadav, A. R. (2012). Solid acid catalyzed solventless highly selective, effective and reusable method for synthesis of 1, 4-dioxanol using glycerol and cyclohexanone. Rev. Chem. Eng., 4(6), 608–619.
Hagui, W., Essid, R., Amri, S., Feris, N., Khabbouchi, M., Tabbene, O., Limam, F., Srasra, E., Besbes, N. (2019). Acid-activated clay as heterogeneous and reusable catalyst for the synthesis of bioactive cyclic ketal derivatives. Turk. J. Chem., 43(2), 435–451. doi:10.3906/kim-1807-58
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

