BIOACTIVE COMPOUNDS EXTRACTED FROM THE HERB SPERANSKIA TUBERCULATA (BUNGE) BAILL
DOI:
https://doi.org/10.15421/jchemtech.v32i3.305125Keywords:
Speranskia tuberculata (Bunge) Baill; ethyl acetate extract; petroleum ether extract; LC/MS; commercially available cancer cell lines; MTT cytotoxicity test.Abstract
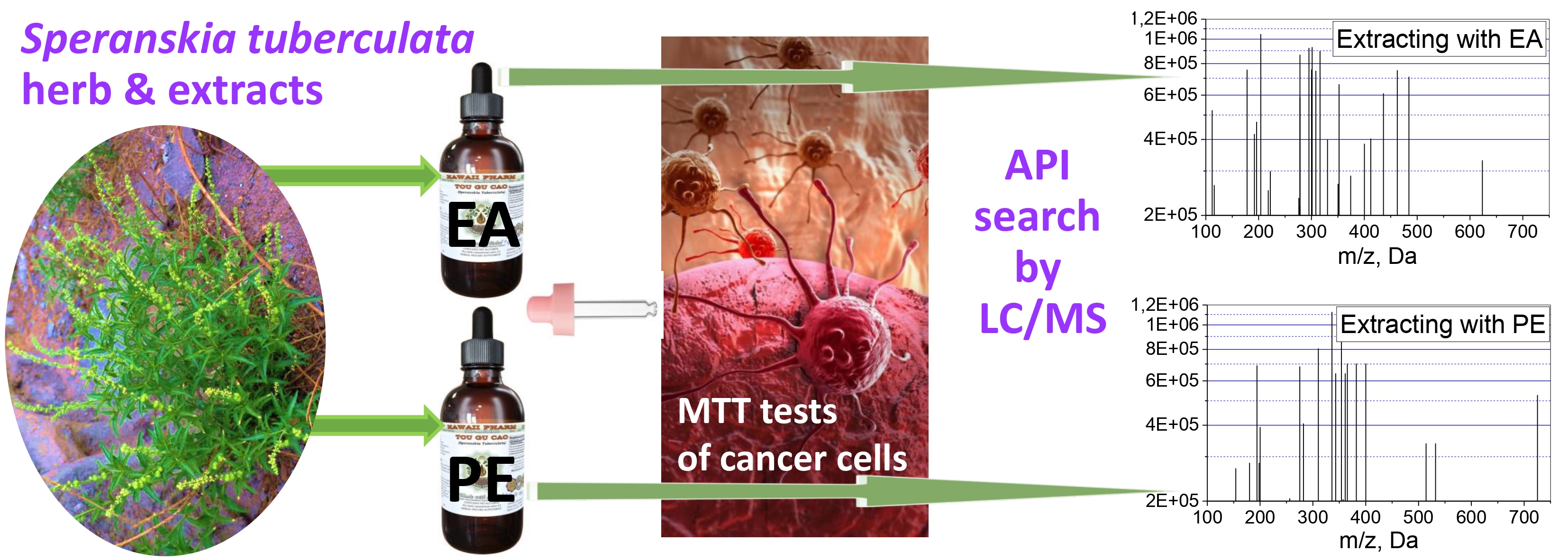
Aim. Identification of bioactive compounds in preparations extracted from the herb Speranskia tuberculata using two different solvents and comparison of the effects of the extracts on human cancer cells of four different lines. Methods. Crude extracts were extracted using ethyl acetate or petroleum ether, followed by distillation, drying and preparation of working solutions based on dimethyl sulfoxide in the 12.5–75 μg/mL concentration range. MTT tests were used to determine the effect of drug concentrations on the viability of cancer cells of four commercially available lines: A549 (lung adenocarcinoma), HEPG2 (hepatocellular carcinoma of the liver), A375 (malignant melanoma) and HELA (pancreatic carcinoma). The tandem liquid chromatography-mass spectroscopy (LC/MS) method was used to identify the main compounds and compare the composition of both extracts. Results. The developed preparations reduced the viability of all 4 types of cancer cells. Ethyl acetate extracts are always more effective than petroleum ether extracts. IC50 of ethyl acetate extract varied between 49–53 μg/mL for all cell lines. Approximately 100–200 compounds have been identified, and their number in petroleum ether extracts is roughly twice as high. Concentrations of 11 compounds significantly increased when switching from extraction with petroleum ether to ethyl acetate. The potential biological activity of identified compounds was analysed based on literature data. Conclusions. The studied preparations reduced the viability of various human cancer cells. Preparations extracted with ethyl acetate were more effective against all types of cells. Hundreds of compounds were identified in the extracts; some anticancer effects were discussed.
References
Wang, Y., Zhang, Q., Chen, Y., Liang, C. L., Liu, H., Qiu, F. and Dai, Z. (2020). Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother., 121, 109570. https://doi.org/10.1016/j.biopha.2019.109570
Zhang, Y., Lou, Y., Wang, J., Yu, C. and Shen, W. (2021). Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front. Immunol., 11, 609705. https://doi.org/10.3389/fimmu.2020.609705
Ye, J.-W., Wu, H.-Y., Fu, M.-J., Zhang, P. and Tian, B. (2021). Insights Into the Significance of the Chinese Loess Plateau for Preserving Biodiversity from the Phylogeography of Speranskia tuberculata (Euphorbiaceae). Front. Plant Sci., 12, 604251. https://doi.org/10.3389/fpls.2021.604251
Jo, H.-G., Seo, J., Choi, S. and Lee, D. (2022). East Asian herbal medicine to reduce primary pain and adverse events in cancer patients: A systematic review and meta-analysis with association rule mining to identify core herb combination. Front. Pharmacol., 12. https://doi.org/10.3389/fphar.2021.800571
Wang, K., Chen, Q., Shao, Y., Yin, S., Liu, C., Liu, Y., Wang, R., Wang, T., Qiu, Y. and Yu, H. (2021). Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother., 133, 111044. https://doi.org/10.1016/j.biopha.2020.111044
Fan, Y., LuLu Zhao, Z.-M., Wang, W., Gao, M., Jia, X., Ouyang, H. He, J. (2020). Antitumor activities and mechanisms of Traditional Chinese medicines formulas: A review. Biomed. Pharmacother., 132, 110820. https://doi.org/10.1016/j.biopha.2020.110820
Niwano, Y., Saito, K., Yoshizaki, F., Kohno, M. Ozawa, T. (2011). Extensive screening for herbal extracts with potent antioxidant properties. J. Clin. Biochem. Nutr., 48(1), 78–84. https://doi.org/10.3164/jcbn.11-013FR
Kim, G., Gan, R.-Y., Zhang, D., Farha, A. K., Habimana, O., Mavumengwana, V., Li, H.-B., Wang, X.-H. and Corke, H. (2020). Large-Scale Screening of 239 Traditional Chinese Medicinal Plant Extracts for Their Antibacterial Activities against Multidrug-Resistant Staphylococcus aureus and Cytotoxic Activities. Pathogens (Basel, Switzerland), 9(3), 185. https://doi.org/10.3390/pathogens9030185
Zhou, Y.-X., Wang, S.-J., Li, Y., Xia, W., Meng, X.-Y., Peng, C. Zhang, H. (2015). Evaluation of analgesic, anti-inflammatory and antipyretic activities of the ethanol extract from Speranskia tuberculate. Afr. J. Tradit., Complementary Altern. Med., 12(3), 49–54. https://doi.org/10.4314/ajtcam.v12i3.6
Li, C., Zhang, C.-Z., Hu, F. D. and Shi, J. G. (2000). [Chemical constituents from Speranskia tuberculata (Bge.) Baill]. Zhongguo Zhong Yao Za Zhi, 25(5), 291–292. [in Chinese].
Yu, S., Yan, H., Zhang, L., Shan, M., Chen, P., Ding, A. Li, S. F. Y. (2017). A review on the phytochemistry, pharmacology, and pharmacokinetics of Amentoflavone, a naturally occurring biflavonoid. Molecules, 22(2), 299. https://doi.org/10.3390/molecules22020299
Li, Y.-M., Zhao, Y.-Y., Fan, Y.-B., Wang, X., Cai, L. N. (1997). Flavonoids from Speranskia Tuberculata. Journal of Chinese Pharmaceutical Sciences, 6, 70–74.
Sun, Z., Derkach, T. M. (2024). Biologically Active Compounds in the Extracts from the Speranskia Tuberculata (Bunge) Baill Herb and Their Effect on the Viability of Cancer Cells of Five Different Lines. Pharmaceutical Review, 1(69), 23–34. https://doi.org/10.11603/2312-0967.2024.1.14441
Wang, Y., Li, C.-H., Luo, B., Sun, Y. N., Kim, Y. H., Wei, A.-Z., Gao, J.-M. (2016). Isobutylhydroxyamides from Zanthoxylum bungeanum and Their Suppression of NO Production. Molecules, 21(10), 1416. https://doi.org/10.3390/molecules21101416
Shobi, T. M., Viswanathan, M. B. G. (2018). Antibacterial activity of di-butyl phthalate isolated from Begonia malabarica. J. Appl Biotechnol Bioeng., 5(2), 101–104. https://doi.org/10.15406/jabb.2018.05.00123
Chen, J., Fan, S., Guo, J., Yang, J., Pan, L. and Xia, Y. (2024). Discovery of anticancer function of Febrifugine: Inhibition of cell proliferation, induction of apoptosis and suppression steroid synthesis in bladder cancer cells. Toxicol. Appl. Pharmacol., 484, 116878. https://doi.org/10.1016/j.taap.2024.116878
Lu, S.-Y., Wang, H.-M., Feng, N., and Ma, A.-I. (2023). Total synthesis of bi-magnolignan. RSC Adv., 13(13), 8844–8846. http://dx.doi.org/10.1039/D3RA01121F
Dugasani, S., Rao Pichika, M., Nadarajah, V. D., Balijepalli, M. K., Tandra, S. and Korlakunta, J. N. (2010). Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol., 127(2), 515–520. https://doi.org/10.1016/j.jep.2009.10.004
Baptista Moreno Martin, A. C., Tomasin, R., Luna-Dulcey, L. Graminha, A. E., Araújo, Naves, M., Teles, R. H. G., da Silva, V. D., da Silva, J. A., Vieira, P. C., Annabi, B., Cominetti, M. R. (2020). [10]-Gingerol improves doxorubicin anticancer activity and decreases its side effects in triple-negative breast cancer models. Cell Oncol., 43, 915–929. https://doi.org/10.1007/s13402-020-00539-z
Abdul Bari, S., Aizhamal, B., Ho, K.-J., Hyun, L.-Y. & Hun, P.K. (2022). Inhibition of Bacterial Neuraminidase and Biofilm Formation by Ugonins Isolated From Helminthostachys Zeylanica (L.). Front. Pharmacol., 13. https://doi.org/10.3389/fphar.2022.890649
Yamauchi, K., Mitsunaga, T., Itakura, Y. and Batubara, I. (2015). Extracellular melanogenesis inhibitory activity and the structure-activity relationships of ugonins from Helminthostachys zeylanica roots. Fitoterapia, 104, 69–74. https://doi.org/10.1016/j.fitote.2015.05.006
Grabarczyk, M., Wińska, K., Mączka, W., Potaniec, B., Anioł, M. (2015). Loliolide - the most ubiquitous lactone. Acta Universitatis Lodziensis. Folia Biologica Et Oecologica, 11, 1–8. https://doi.org/10.1515/fobio-2015-0001
Antika, L., Tasfiyati, A., Hikmat, H., Septama, A. (2022). Scopoletin: a review of its source, biosynthesis, methods of extraction, and pharmacological activities. Zeitschrift für Naturforschung C, 77(7-8), 303–316. https://doi.org/10.1515/znc-2021-0193
Niu, L., Li, W., Chen, X., Su, X., Dong, J., Liao, Q., Zhou, X., Shi, S., Sun, R. (2023). 1-Monopalmitin promotes lung cancer cells apoptosis through PI3K/Akt pathway in vitro. Environ. Toxicol., 38(11), 2621–2631. https://doi.org/10.1002/tox.23897
Tsivileva, O. M., Koftin, O. V. and Evseeva, N. V. (2022). Coumarins as Fungal Metabolites with Potential Medicinal Properties. Antibiotics (Basel, Switzerland), 11(9), 1156. https://doi.org/10.3390/antibiotics11091156
Hosseini, S. H., Masullo, M., Cerulli, A., Martucciello, S., Ayyari, M., Pizza, C. and Piacente, S. (2019). Antiproliferative Cardenolides from the Aerial Parts of Pergularia tomentosa. J. Nat. Prod., 82(1), 74–79. https://doi.org/10.1021/acs.jnatprod.8b00630
Zoya, M., Rabea, P., Bushra, P., Sultan, Z., Mohammad, A. K., Asifa, K., Sheersh, M., Sayeed, A. and Syed, A. H. (2021). Anticancer potential of andrographolide from Andrographis paniculata (Burm.f.) Nees and its mechanisms of action. J. Ethnopharmacol., 272, 113936. https://doi.org/10.1016/j.jep.2021.113936
Indira, M., Peele, K. A., Krupanidhi, S., Prabhakar, K. V., Vimala, K. B. S., Kavya, P. S., Sravya, I. and Venkateswarulu, T. C. (2023). In Vitro Assessment of The Bioactive Compounds and Anticancer Potential of Citrus medica Leaf Extract. Trop. Life Sci. Res., 34(3), 197–215. https://doi.org/10.21315/tlsr2023.34.3.11
Hidayat, A., Turjaman, M., Qamyari, R., Imanuddin, R., Tohir, D., Rahmanto, R. G. H. and Susilowati, A. (2021). Bioactive composition, antifungal, antioxidant, and anticancer potential of agarwood essential oil from decaying logs (Gyrinops spp.) of Papua Island (Indonesia). J. Appl. Pharm. Sci., 11(10), 070–078. https://doi.org/10.7324/JAPS.2021.1101010
Hajdú, Z., Hohmann, J., Forgo, P., Máthé, I., Molnár, J. and Zupkó, I. (2014). Antiproliferative activity of Artemisia asiatica extract and its constituents on human tumor cell lines. Planta medica, 80(18), 1692–1697. https://doi.org/10.1055/s-0034-1383146
Chen, Y., Cheng, Q.-Z., Lv, S., Kang, Z. and Zeng, S. (2023). Advances in the phytochemistry and pharmacology of plant-derived phthalides. Heliyon, 9(12), e22957. https://doi.org/10.1016/j.heliyon.2023.e22957
Sharma, G. V. M., Rajagopal, D. and Rao, E. S. (1989). A Practical Synthesis of (2E,6E,8E)-N-(2-Methylpropyl)-2,6,8-hexadecatrien-10-ynamide. Synth. Commun., 19(18), 3181–3189. https://doi.org/10.1080/00397918908052718
Shu, B., Ying, J., Wang, T., Xia, M., Zhao, W. and You, L. (2019). Microbiota and Chemical Compounds in Fermented Pinelliae Rhizoma (Banxiaqu) from Different Areas in the Sichuan Province, China. Pol. J. Microbiol., 68(1), 83–92. https://doi.org/10.21307/pjm-2019-010
Neeraj, T., Arun, K., Ashish, K. S., Surabhi, B., Anand, K. A., Dhiraj, K., Vinod, K. T. and Rakesh, K. S. (2019). 8 - Leishmaniasis control: limitations of current drugs and prospects of natural products. In: G. Brahmachari (Ed.), Natural Product Drug Discovery. Discovery and Development of Therapeutics from Natural Products Against Neglected Tropical Diseases, 293–350. https://doi.org/10.1016/B978-0-12-815723-7.00008-0
Götz, M. E., Eisenreich, A., Frenzel, J., Sachse, B. and Schäfer, B. (2023). Occurrence of Alkenylbenzenes in Plants: Flavours and Possibly Toxic Plant Metabolites. Plants, 12, 2075. https://doi.org/10.3390/plants12112075
Wong, S.-M., Pezzuto, J. M., Fong, H. H. S., and Farnsworth, N. R. (1985). Isolation, Structural Elucidation, and Chemical Synthesis of 2-Hydroxy-3-octadecyl-5-methoxy-1,4-benzoquinone (Irisoquin), a Cytotoxic Constituent of Iris missouriensis. Journal of Pharmaceutical Sciences, 74(10), 1114–1116. https://doi.org/10.1002/jps.2600741023
Wu, T., Liu, X., Sun, Z., Xing, S., Han, L., Li, X., Pan, X., Chen, J., Zhou, M., Derkach, T. and Bielicki, J. K. (2022). Fruit of Phyllanthus emblica L. suppresses macrophage foam-cell genesis and vascular lipid deposition using in vivo and in vitro models of early atherosclerosis development. Food Sci. Technol. Res., 28(4), 317–328. https://doi.org/10.3136/fstr.FSTR-D-22-00002
Batiha, G. E., Teibo, J. O., Wasef, L., Shaheen, H. M., Akomolafe, A. P., Teibo, T. K. A., Al-Kuraishy, H. M., Al-Garbeeb, A. I., Alexiou, A. and Papadakis, M. (2023). A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg's Arch. Pharmacol., 396(5), 877–900. https://doi.org/10.1007/s00210-023-02392-x
Museum of Materia Medica. Institute of Natural Medicine, University of Toyama, Japan (2024). The Data Base of Ethno-medicines in the world. Crude drug sample database. https://ethmed.toyama-wakan.net/SearchEn/View/36423
Haruya, T., Kosuke, K., Tsuyoshi, G., Hideyuki, H., Shinsuke, M., Hideyuki, S., Daisuke, Sh., Rieko, N., Hiroyasu, I., Nobuyuki, T. and Teruo, K. (2015). 9‐Oxo‐10(E), 12(Z),15(Z)‐Octadecatrienoic Acid Activates Peroxisome Proliferator‐Activated Receptor α in Hepatocytes. Lipids, 50(11), 1083–1091. https://doi.org/10.1007/S11745-015-4071-3
Tian, C., Gao, X., Yang, J., Guo, Y., Wang, H. and Liu, M. (2018). Chemical compositions, extraction technology, and antioxidant activity of petroleum ether extract from Abutilon theophrasti Medic. leaves. Int. J. Food Prop., 21(1), 1789–1799. https://doi.org/10.1080/10942912.2018.1494198
National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 3083655, 2-(1H-imidazol-5-yl)ethanol. https://pubchem.ncbi.nlm.nih.gov/compound/2-_1H-imidazol-5-yl_ethanol.
Kwon, S.-S., Kim, J.-H., Jeong, H.-U., Cho, Y.-Y., Oh, S.-R., Lee, H.-S. (2016). Inhibitory Effects of Aschantin on Cytochrome P450 and Uridine 5′-diphospho-glucuronosyltransferase Enzyme Activities in Human Liver Microsomes. Molecules, 21, 554. https://doi.org/10.3390/molecules21050554
Yang, Z.-D., Duan, D.-Z. (2012). A new alkaloid from Fritillaria ussuriensis Maxim. Fitoterapia, 83(1), 137–141. https://doi.org/10.1016/j.fitote.2011.10.006
Faisal, M., Sarker, M., Rahman, A., Hossain, A., Rahman, S., Bashar, A., Jahan, R. and Rahmatullah, M. (2014). Murraya paniculata (L.) Jack: A Potential Plant for Treatment of Toothache. Symbiosis, 2, 1–3. https://doi.org/10.15226/jdodt.2014.00123
Derkach, T. M. and Starikova, O. O. (2019). Variation of chemical composition of medicinal herbs of different producers. Journal of Chemistry and Technologies, 27(1), 79–91. https://doi.org/10.15421/091909
Snyder, L. R. (1978). Classification of the Solvent Properties of Common Liquids. J. Chromatogr. Sci., 16(6), 223–234. https://doi.org/10.1093/chromsci/16.6.223
Nawaz, H., Shad, M. A., Rehman, N., Andaleeb, H. and Ullah, N. (2020). Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci., 56, e17129. https://doi.org/10.1590/s2175-97902019000417129
Ounis, R., Benchikh, F., Amira, S., Benabdallah, H., Amira, H., Mamache, W., Bensouici, C., and Hellal, K. (2023). Impact of Extraction Solvent Polarity: Antioxidant Activity of Methanolic, Hydromethanolic and Aqueous Decocted Extracts of Algerien Thymelaea hirsuta (L.) Endl. Areal Parts. Turk. J. Agric. - Food Sci. Technol., 11(6), 1161–1167. https://doi.org/10.24925/turjaf.v11i6.1161-1167.5931
Zarrinmehr, M. J., Daneshvar, E., Nigam, S., Gopinath, K. P., Biswas, J. K., Kwon, E. E., Wang, H., Farhadian, O. Bhatnagar, A. (2022). The effect of solvents polarity and extraction conditions on the microalgal lipids yield, fatty acids profile, and biodiesel properties. Bioresource Technology (B), 344, 126303. https://doi.org/10.1016/j.biortech.2021.126303
Djoumbou Feunang, Y., Eisner, R., Knox, C., Chepelev, L., Hastings, J., Owen, G., Fahy, E., Steinbeck, C., Subramanian, S., Bolton, E., Greiner, R., Wishart, D. S. (2016). ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. J. Cheminf., 8, 61. https://doi.org/10.1186/s13321-016-0174-y
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

