SYNTHESIS, CRYSTAL STRUCTURE, THERMAL STABILITY AND HIRSHFELD SURFACE ANALYSIS OF AZAMETALLOCROWN COPPER(II) COMPOUNDS WITH 4-IODOPYRAZOLE
DOI:
https://doi.org/10.15421/jchemtech.v32i3.305413Keywords:
copper, copper complexes, crystal structure, pyrazole, X-ray structure analysis, metallacrown, thermal analysis, Hirshfeld surface analysisAbstract
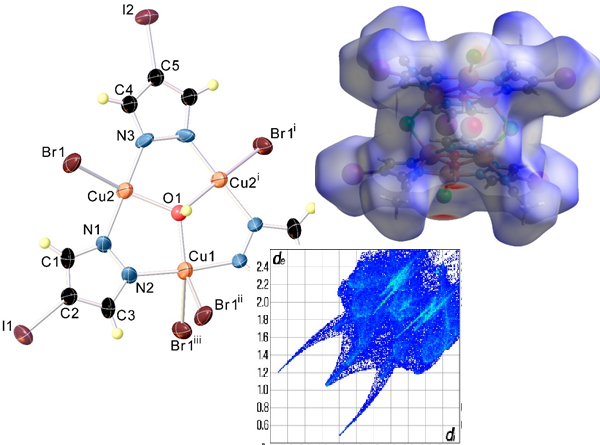
The reaction of copper(II) chloride and copper(II) bromide with 4-iodopyrazole (HIpz or C3H3IN2) in the presence of copper powder in acetonitrile leads to the formation of the corresponding trinuclear copper(II) pyrazolates, [Cu3(OH)(C3H2IN2)3Cl2 ·2CH3CN]2 (1), and [Cu3(OH)(C3H2IN2)3Br2]n (2), respectively. Both complexes maintain the [Cu₃(μ₃-OH)(μ-Ipz)₃]²⁺ core, but they exhibit relevant differences in their molecular structures, as well as in their supramolecular arrangements. The crystal structure of complex 1 comprises trinuclear 9-azaMC-3 units linked by chlorine anions into dimers and arranged into polymeric chains through hydrogen bonds formed between the hydrogen atoms of the OH group and the Cl⁻ anions of adjacent fragments. The crystal structure of complex 2 is built up from the parallel packing of supramolecular chains running along the c-axis direction. Within each chain, the trinuclear metallacrown-like complex subunits are interconnected by bridging bromine atoms. Analysis of the geometry of the polyhedra revealed that the coordination polyhedron geometry of the pentacoordinated copper(II) ions in both structures is intermediate between trigonal bipyramidal and square pyramidal. The process of thermal degradation of the synthesized complexes with metallocrown structure was studied with the help of differential thermal analysis and thermogravimetry. Hirshfeld surfaces analysis was performed to investigate the intermolecular atomic contacts in the crystal structure of the title complexes.
References
Corrochano-Monsalve, M., González-Murua, C., Bozal-Leorri, A., Lezama, L., Artetxe, B. (2021). Mechanism of action of nitrification inhibitors based on dimethylpyrazole: A matter of chelation. Science of The Total Environment, 752, 141885. https://doi.org/10.1016/j.scitotenv.2020.141885
Roy, M., Pal, A. K., Adhikary, A., Datta, A., Mondal, R. (2020). Paradoxical design of a serendipitous pyrazolate bridging mode: a pragmatic strategy for inducing ineluctable ferromagnetic coupling. Dalton Transactions, 49(39), 13704–13716. https://doi.org/10.1039/D0DT02468F
Davydenko, Yu. M., Vitske, V. A., Pavlenko, V. A., Haukka, M., Vynohradov, O. S., Fritsky, I. O. (2022). Synthesis, crystal structure and properties of coordination polymers based on (3,5-dimethyl-1Н-pyrazole-4-yl)-acetic acid. Journal of Chemistry and Technologies, 30(2), 174–183. https://doi.org/10.15421/jchemtech.v30i2.252517
Davydenko, Y., Pavlenko, V., Fritsky, I., Vynohradov, O. (2022). Synthesis, x-ray crystal structure, spectroscopic characterization and hirshfeld surface analysis of dichloro-bis (3,5-dimethyl-4-amino-1h-pyrazole) cobalt (II). Ukrainian Chemistry Journal, 88(6), 127–136. https://doi.org/10.33609/2708-129X.88.06.2022.127-136
Mandal, N. K., Nandi, S., Souilamas, S. B., Garcia, C. J. G., Acharya, K., Naskar, J. P. (2024). Design, synthesis and structure of a trinuclear copper (II) complex having Cu3OH core with regard to aspects of antiproliferative activity and magnetic properties. New Journal of Chemistry. doi: 10.1039/D3NJ04859D
Cañón-Mancisidor, W., Hermosilla-Ibáñez, P., Spodine, E., Paredes-García, V., Gómez-García, C. J., & Venegas-Yazigi, D. (2023). Spin Frustrated Pyrazolato Triangular CuII Complex: Structure and Magnetic Properties, an Overview. Magnetochemistry, 9(6), 155. https://doi.org/10.3390/magnetochemistry9060155
Di Nicola, C., Karabach, Y. Y., Kirillov, A. M., Monari, M., Pandolfo, L., Pettinari, C., & Pombeiro, A. J. L. (2007). Supramolecular assemblies of trinuclear triangular copper (II) SBUs through hydrogen bonds. Generation of different MOFs, valuable catalysts for peroxidative oxidation of alkanes. Inorganic chemistry, 46, 221–230. https://doi.org/10.1021/ic061595n
Mezei, G., Rivera-Carrillo, M., Raptis, R. G. (2004). Effect of copper-substitution on the structure and nuclearity of Cu (II)-pyrazolates: from trinuclear to tetra-, hexa-and polynuclear complexes. Inorganica chimica acta, 357(12), 3721–3732. https://doi.org/10.1016/j.ica.2004.05.022
Solomon, E. I., Sundaram, U. M., Machonkin, T. E. (1996). Multicopper oxidases and oxygenases. Chemical reviews, 96(7), 2563–2606. https://doi.org/10.1021/cr950046o
Mezei, G., McGrady, J. E., Raptis, R. G. (2005). First Structural Characterization of a Delocalized, Mixed-Valent, Triangular Cu37+ Species: Chemical and Electrochemical Oxidation of a CuII3 (μ3-O) Pyrazolate and Electronic Structure of the Oxidation Product. Inorganic chemistry, 44(21), 7271–7273. https://doi.org/10.1021/ic050729e
Rivera-Carrillo, M., Chakraborty, I., Mezei, G., Webster, R. D., & Raptis, R. G. (2008). Tuning of the [Cu3 (μ-O)] 4+/5+ redox couple: Spectroscopic evidence of charge delocalization in the mixed-valent [Cu3 (μ-O)] 5+ species. Inorganic chemistry, 47(17), 7644–7650. https://doi.org/10.1021/ic800531y
Scatena, R., Massignani, S., Lanza, A. E., Zorzi, F., Monari, M., Nestola, F., Pandolfo, L. (2021). Synthesis of Coordination Polymers and Discrete Complexes from the Reaction of Copper (II) Carboxylates with Pyrazole: Role of Carboxylates Basicity. Crystal Growth & Design, 22(2), 1032–1044. https://doi.org/10.1021/acs.cgd.1c00861?urlappend=%3Fref%3DPDF&jav=VoR&rel=cite-as
Zaleski, C. M. (Ed.). (2022). Advances in metallacrown chemistry. Springer Nature.
Zhang, H. G., Du, Y. C., Yang, H., Zhuang, M. Y., Li, D. C., Dou, J. M. (2019). A new family of {Co4Ln8} metallacrowns with a butterfly-shaped structure. Inorganic Chemistry Frontiers, 6(7), 1904–1908. https://doi.org/10.1039/C9QI00661C
Elguero, J., Alkorta, I. (2020). A computational study of metallacycles formed by pyrazolate ligands and the coinage metals M= Cu (I), Ag (I) and Au (I):(pzM) n for n= 2, 3, 4, 5 and 6. Comparison with structures reported in the Cambridge Crystallographic Data Center (CCDC). Molecules, 25(21), 5108. https://doi.org/10.3390/molecules25215108
Tian, Y., Wang, Z. Y., Zang, S. Q., Li, D., Mak, T. C. (2019). Luminescent cyclic trinuclear coinage metal complexes with aggregation-induced emission (AIE) performance. Dalton Transactions, 48(7), 2275–2279. https://doi.org/10.1039/C8DT04898C
Davydenko, Y. M., Vynohradov, O. S., Pavlenko, V. A., & Fritsky, I. O. (2023). Synthesis, structural and spectroscopic characterizations, hirshfeld surface analysis of two coordination compounds assembled from copper and carboxylates, 3, 5-dimethyl-1h-pyrazole. Journal of Chemistry and Technologies, 31(3), 468–476. https://doi.org/10.15421/jchemtech.v31i3.276647
Vynohradov, O. S., Davydenko, Y. M., Pavlenko, V. O., Naumova, D. D., Fritsky, I. O., Shova, S., & Prysiazhna, O. V. (2023). CuBr2 as a bromination agent of pyrazole-based ligand: synthesis of copper (II) coordination compounds by oxidative dissolution of copper powder in organic solvents. Journal of Chemistry and Technologies, 31(3), 493–506. https://doi.org/10.15421/jchemtech.v31i3.281190
Szymańska, I. B., Madajska, K., Butrymowicz, A., Barwiołek, M. (2021). Copper (II) Perfluorinated Carboxylate Complexes with Small Aliphatic Amines as Universal Precursors for Nanomaterial Fabrication. Materials, 14(23), 7451. https://doi.org/10.3390/ma14237451
Shi, K., Mathivathanan, L., Boudalis, A. K., Turek, P., Chakraborty, I., Raptis, R. G. (2019). Nitrite Reduction by Trinuclear Copper Pyrazolate Complexes: An Example of a Catalytic, Synthetic Polynuclear NO Releasing System. Inorganic Chemistry, 58(11), 7537–7544. https://doi.org/10.1021/acs.inorgchem.9b00748
Domasevitch, K. V., Lysenko, A. B. (2019). Crystal structure of poly [[(μ3-hydroxido-κ3O: O: O)(μ3-selenato-κ3O1: O2: O3) tris [μ3-2-(1, 2, 4-triazol-4-yl) acetato-κ3N1: N2: O] tricopper (II)] dihydrate]. Acta Crystallographica Section E: Crystallographic Communications, 75(8), 1185–1189. https://doi.org/10.1107/S2056989019009812
Chen, J. H., Wei, D., Yang, G., Ma, J. G., Cheng, P. (2020). A systematic investigation of structural transformation in a copper pyrazolato system: a case study. Dalton Transactions, 49(4), 1116-1123. https://doi.org/10.1039/C9DT04263F
Davydenko, Y. M., Demeshko, S., Pavlenko, V. A., Dechert, S., Meyer, F., Fritsky, I. O. (2013). Synthesis, Crystal Structure, Spectroscopic and Magnetically Study of Two Copper(II) Complexes with Pyrazole Ligand. Zeitschrift Für Anorganische Und Allgemeine Chemie, 639(8-9), 1472–1476. https://doi.org/10.1002/zaac.201300078
Casarin, M., Corvaja, C., Di Nicola, C., Falcomer, D., Franco, L., Monari, M., Piccinelli, F. (2005). One-dimensional and two-dimensional coordination polymers from self-assembling of trinuclear triangular Cu (II) secondary building units. Inorganic chemistry, 44(18), 6265–6276. https://doi.org/10.1021/ic050678l
Casarin, M., Cingolani, A., Di Nicola, C., Falcomer, D., Monari, M., Pandolfo, L., Pettinari, C. (2007). The different supramolecular arrangements of the triangular [Cu3 (mu3-OH)(mu-pz) 3] 2+ SBUs. Synthesis of a porous coordination polymer with permanent hexagonal channels. Crystal growth & design, 7, 676–685. https://doi.org/10.1021/cg060501h
Massignani, S., Scatena, R., Lanza, A., Monari, M., Condello, F., Nestola, F., Pandolfo, L. (2017). Coordination polymers from mild condition reactions of copper (II) carboxylates with pyrazole (Hpz). Influence of carboxylate basicity on the self-assembly of the [Cu3 (μ3-OH)(μ-pz)3]2+ secondary building unit. Inorganica Chimica Acta, 455, 618–626. https://doi.org/10.1016/j.ica.2016.05.009
Ahmed, B. M., Mezei, G. (2016). From ordinary to extraordinary: Insights into the formation mechanism and pH-dependent assembly/disassembly of nanojars. Inorganic Chemistry, 55(15), 7717–7728. https://doi.org/10.1021/acs.inorgchem.6b01172
Sheldrick, G. M. (2015). Acta Crystallogr., Sect. A: Found. Adv. https://doi.org/10.1107/S2053273314026370
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A., Puschmann, H. (2009). OLEX2: a complete structure solution, refinement and analysis program. Journal of applied crystallography, 42(2), 339–341. https://doi.org/10.1107/S0021889808042726
Sheldrick, G. M. (2015). Acta Crystallogr., Sect. C. Struct. Chem., 71, 3. https://doi.org/10.1107/S2053229614024218
Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J., Verschoor, G. C. (1984). Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper (II) perchlorate. Journal of the Chemical Society, Dalton Transactions, (7), 1349–1356. https://doi.org/10.1039/dt9840001349
Llunell, M., Casanova, D., Cirera, J., Alemany, P., Alvarez, S. (2013). SHAPE, version 2.1. Universitat de Barcelona, Barcelona, Spain, 2103.
Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D., Spackman, M. A. (2021). It CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. Journal of Applied Crystallography, 54(3), 1006–1011. https://doi.org/10.1107/S1600576721002910
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

