A COMPUTATIONAL EVALUATION OF STRUCTURES AND PROPERTIES OF NUCLEIC ACID BASE – WATER COMPLEXES
DOI:
https://doi.org/10.15421/jchemtech.v33i1.307766Keywords:
DNA bases, DFT study, electron affinity, ionization potential, binding energy, hydration shell, radiationAbstract
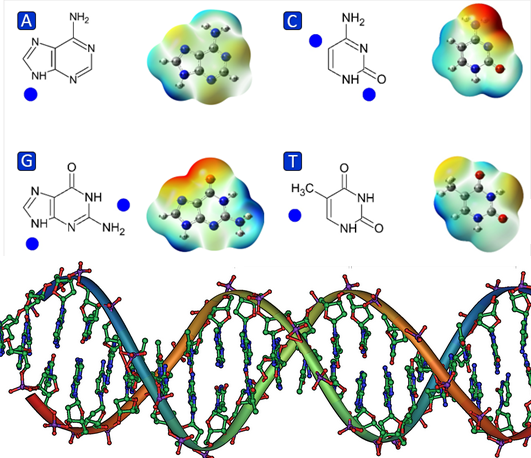
Electronic properties, electron affinity and ionization potential, binding energies, and conformational changes of neutral, cationic, and anionic DNA base-water complexes, [Base (H2O)n] (0,+) (n = 0, 4, 8, 14) have been investigated in gas and aqueous phases using the DFT/M06-2X hybrid functional method with 6-31++g(d,p) basis set implemented in the Gaussian09 software package. We find that the electronic properties of DNA bases are strongly influenced by implicit and explicit solvation. While purines show high electron affinity, pyrimidines show the greatest ionization potential at the hydration levels observed. Data also reveals that binding energy lowers in implicit solvation. Additionally, the molecular electrostatic potential surfaces of each compound were calculated to identify the most favorable sites for water binding. The ESP charges, derived from the electron density, indicate that regions of the highest electronegativity do not always correspond to the lowest charge, suggesting complex interactions in the solvation processes. These results provide significant insight into the effects of hydration on the electrostatic properties of DNA nucleobases.
References
Russo, N., Toscano, M., Grand, A. (2000). Theoretical determination of electron affinity and ionization potential of DNA and RNA bases., Journal of Computational Chemistry, 21(14), 1243–1250. doi:10.1002/1096-987x(20001115)21:14<1243::aid-jcc3>3.0.co;2-m
Kumar, Anil; Mishra, P. C.; Suhai, Sándor (2005). Adiabatic Electron Affinities of the Polyhydrated Adenine−Thymine Base Pair: A Density Functional Study. The Journal of Physical Chemistry A, 109(17), 3971–3979. doi:10.1021/jp0456178
Rawtani, Deepak; Kuntmal, Binal; Agrawal, Y. (2016). Charge transfer in DNA and its diverse modelling approaches. Frontiers in Life Science, 9(3), 214–225. doi:10.1080/21553769.2016.1207570
Hanus, Michal; Ryjáček, Filip; Kabeláč, Martin; Kubař, Tomáš; Bogdan, Tetyana V.; Trygubenko, Semen A.; Hobza, Pavel (2003). Correlated ab Initio Study of Nucleic Acid Bases and Their Tautomers in the Gas Phase, in a Microhydrated Environment and in Aqueous Solution. Guanine: Surprising Stabilization of Rare Tautomers in Aqueous Solution. Journal of the American Chemical Society, 125(25), 7678–7688. doi:10.1021/ja034245y
Krueger, A. T., Kool, E. T. (2007). Model systems for understanding DNA base pairing. Current Opinion in Chemical Biology, 11(6), 588-594. doi:10.1016/j.cbpa.2007.09.019
Dekker, Cees; Ratner, Mark (2001). Electronic properties of DNA. Physics World, 14(8), 29–33. doi:10.1088/2058-7058/14/8/33
Lewis, Kristen; Copeland, Kari; Hill, Glake (2014). One-electron redox properties of DNA nucleobases and common tautomers. International Journal of Quantum Chemistry, 114(24), 1678–1684. doi:10.1002/qua.24745
Smyth, Maeve; Kohanoff, Jorge (2011). Excess Electron Localization in Solvated DNA Bases. Physical Review Letters, 106(23), 238108. doi:10.1103/PhysRevLett.106.238108
Baiocco, G.; Giraudo, M.; Bocchini, L.; Barbieri, S.; Locantore, I.; Brussolo, E.; Giacosa, D.; Meucci, L.; Steffenino, S.; Ballario, A.; Barresi, B.; Barresi, R.; Benassai, M.; Ravagnolo, L.; Narici, L.; Rizzo, A.; Carrubba, E.; Carubia, F.; Neri, G.; Crisconio, M.; Piccirillo, S.; Valentini, G.; Barbero, S.; Giacci, M.; Lobascio, C.; Ottolenghi, A. (2018). A water-filled garment to protect astronauts during interplanetary missions tested on board the ISS. Life Sciences in Space Research, 18, 1–11. doi:10.1016/j.lssr.2018.04.002
Park, Y., Peoples, A. R., Madugundu, G. S., Sanche, L., Wagner, J. R. (2013). Side-by-Side Comparison of DNA Damage Induced by Low-Energy Electrons and High-Energy Photons with Solid TpTpT Trinucleotide. The Journal of Physical Chemistry B, 117(35), 10122–10131. doi:10.1021/jp405397m
Khistyaev, K., Bravaya, K. B., Kamarchik, E., Kostko, O., Ahmed, M., Krylov, A. I. (2011). The effect of microhydration on ionization energies of thymine. FaradayDiscussions,150(),313 doi:10.1039/c0fd00002g
Laage, D., Elsaesser, T., Hynes, J. T. (2017). Water Dynamics in the Hydration Shells of Biomolecules. Chemical Reviews, doi:10.1021/acs.chemrev.6b00765
Pizzino, Gabriele; Irrera, Natasha; Cucinotta, Mariapaola; Pallio, Giovanni; Mannino, Federica; Arcoraci, Vincenzo; Squadrito, Francesco; Altavilla, Domenica; Bitto, Alessandra (2017). Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity, 2017, 1–13. doi:10.1155/2017/8416763
Sevilla, M. D., Becker, D., Kumar, A., Adhikary, A., (2016). Gamma and Ion-Beam Irradiation of DNA: Free Radical Mechanisms, Electron Effects, and Radiation Chemical Track Structure. Radiation Physics and Chemistry. doi:10.1016/j.radphyschem. 2016.04.022.
Blakely, E. A. (2000). Biological Effects of Cosmic Radiation: Deterministic and Stochastic. Health Physics, 79(5), 495–506. doi:10.1097/00004032-200011000-00006
Lyndon, B. (2016). Johnson Space Center. Evidence Report: Risk of Cardiovascular Disease and Other Degenerative Tissue Effects from Radiation Exposure. Houston, Texas: National Aeronautics and Space Administration. https://ntrs.nasa.gov/search.jsp?R=20150016006
Maalouf, M., Durante, M., Foray, N. (2011). Biological Effects of Space Radiation on Human Cells: History, Advances and Outcomes. Journal of Radiation Research, 52(2), 126–146. doi:10.1269/jrr.10128
Cucinotta, F. A.; Kim, M.-H. Y.; Willingham, V., George, K.A. (2008). Physical and Biological Organ Dosimetry Analysis for International Space Station Astronauts. Radiation Research, 170(1), 127–138. doi:10.1667/RR1330.1
Shweta, H., Sen, S. (2018). Dynamics of water and ions around DNA: What is so special about them? Journal of Biosciences. doi:10.1007/s12038-018-9771-4
Garrett, B.C., Dixon, D. A., Camaioni, D.M., Chipman, D.M., Johnson, M. A., Jonah, C. D., Zwier, T. S. (2005). Role of Water in Electron-Initiated Processes and Radical Chemistry: Issues and Scientific Advances. Chemical Reviews, 105(1), 355–390. doi:10.1021/cr030453x
Garrett, B.C., Colson, S.D., Dixon, D.A., Laufer, A.H, Ray, D. (2003). Understanding the Role of Water on Electron-Initiated Processes and Radical Chemistry. United States. Web.
Shukla, M.K., Leszczynski, J. (2008). Radiation Induced Molecular Phenomena In Nucleic Acids: A Brief Introduction. In: Shukla, M.K., Leszczynski, J. (eds) Radiation Induced Molecular Phenomena in Nucleic Acids. Challenges and Advances In Computational Chemistry and Physics, 5. Springer, Dordrecht. doi: 10.1007/978-1-4020-8184-2_1
Sadr-Arani, L., Mignon, P., Chermette, H., Abdoul-Carime, H., Farizon, B., Farizon, M., (2015). Fragmentation mechanisms of cytosine, adenine and guanine ionized bases. Phys. Chem. Chem. Phys., 17(17), 11813–11826. doi:10.1039/c5cp00104h
Khistyaev, Kirill; Bravaya, Ksenia B.; Kamarchik, Eugene; Kostko, Oleg; Ahmed, Musahid; Krylov, Anna I. (2011). The effect of microhydration on ionization energies of thymine. Faraday Discussions, 150, 313. doi:10.1039/c0fd00002g.
Brovchenko, I., Krukau, A., Oleinikova, A., Mazur, A. K. (2006). Water Percolation Governs Polymorphic Transitions and Conductivity of DNA. Physical Review Letters, 97(13), 137801. doi:10.1103/PhysRevLett.97.137801
Shishkin, O., Gorb, L., Leszczynski, J. (2000). Modeling of the Hydration Shell of Uracil and Thymine. International. Journal of Molecular Sciences, 1(2), 17–27. doi:10.3390/ijms1020017
Danilov, V. I., van Mourik, T., Poltev, V. I. (2006). Modeling of the “hydration shell” of uracil and thymine in small water clusters by DFT and MP2 methods. Chemical Physics Letters, 429(1-3), 255–260. doi:10.1016/j.cplett.2006.08.035
Stupar, M., Vidovic, V., Lukac, D. (2011). Functions of human non-coding DNA sequences, Archive of Oncology, 19(3-4), 81–85. doi:10.2298/aoo1104081s
Sundaralingam, М., Pan, В. (2002). Hydrogen and hydration of DNA and RNA oligonucleotides, Biophysical Chemistry, 95(3), 273–282. doi:10.1016/s0301-4622(01)00262-9
Schneider, B.; Cohen, D.M.; Schleifer, L.; Srinivasan, A.R.; Olson, W.K.; Berman, H.M. (1993). A systematic method for studying the spatial distribution of water molecules around nucleic acid bases. Biophysical Journal, 65(6), 2291–2303. doi:10.1016/s0006-3495(93)81306-7
Pullman, A. (1980). The Supermolecule Approach to the Solvation Problem. In: Daudel, R., Pullman, A., Salem, L., Veillard, A. (eds) Quantum Theory of Chemical Reactions. Quantum Theory Chemical Reactions, 2. Springer, Dordrecht. doi:10.1007/978-94-010-9716-1_1
Hush, N. S., Cheung, A. S. (1975). Ionization potentials and donor properties of nucleic acid bases and related compounds. Chemical Physical Letters, 34(1), 11–13. doi:10.1016/0009-2614(75)80190-4
Padva, A., O'Donnell, T.J., Lebreton, P.R. (1976). UV photoelectron studies of biological pyrimidines: the valence electronic structure of methyl substituted uracils. Chemical Physical Letters, 41(2), 278–282. doi:10.1016/0009-2614(76)80810-x
Dougherty, D., McGlynn, S. P. (1977). Photoelectron spectroscopy of carbonyls. Biological molecules. The Journal of Chemical Physics, 67(3), 1289. doi:10.1063/1.434949
Dougherty, D., Younathan, E.S., Voll, R., Abdulnur, S., McGlynn, S.P. (1978). Photoelectron spectroscopy of some biological molecules. JournaI of Electron Spectroscopy and Related Phenomena, 13(3), 379–393. doi:10.1016/0368-2048(78)85042-7
Yu, C., Peng, S., Akiyama, I., Lin, J., LeBreton, P. R. (1978). Ultraviolet photoelectron studies of biological pyrimidines. The valence electronic structure of cytosine. Journal of the American Chemical Society, 100(8), 2303–2307. doi:10.1021/ja00476a006
Lin, J., Yu, C., Peng, S., Akiyama, I., Li, K., Lee, Li, K., LeBreton, P. R. (1980). Ultraviolet photoelectron studies of the ground-state electronic structure and gas-phase tautomerism of hypoxanthine and guanine. The Journal of Physical Chemistry, 84(9), 1006–1012. doi:10.1021/j100446a015
Lin, J.; Yu, C.; Peng, S.; Akiyama, I.; Li, K.; Lee, Li Kao; LeBreton, P. R. (1980). Ultraviolet photoelectron studies of the ground-state electronic structure and gas-phase tautomerism of purine and adenine. Journal of the American Chemical Society, 102(14), 4627–4631. doi:10.1021/ja00534a010
Shigeyuki, U., Xu, Y., LeBreton, P. R. (1989). UV photoelectron and quantum mechanical characterization of DNA and RNA bases: valence electronic structures of adenine, 1,9-dimethyl-guanine, 1-methylcytosine, thymine and uracil. Journal of Molecular Structure, 214, 315–328. doi:10.1016/0022-2860(89)80020-1
Wiley, J.R., Robinson, J.M., Ehdaie, S., Chen, E.C.M., Chen, E.S.D., Wentworth, W.E. (1991). The determination of absolute electron affinities of the purines and pyrimidines in DNA and RNA from reversible reduction potentials. Biochemical And Biophysical Research Communication, 180(2), 845. doi:10.1016/s0006-291x(05)81141-6
Ma, J., Wang, F., Mostafavi, M. Ultrafast Chemistry of Water Radical Cation, H₂O(•+), in Aqueous Solutions. Molecules, 23, 244. doi:10.3390/molecules23020244
Von Sonntag, C. (2006). Free-Radical-Induced DNA Damage and Its Repair A Chemical Perspective. Springer. doi:10.1007/3-540-30592-0.
Herbert, John M.; Coons, Marc P. (2017). The Hydrated Electron. Annual Review of Physical Chemistry, 68(1), 447–472. doi:10.1146/annurev-physchem-052516-050816
Kočišek, Jaroslav; Sedmidubská, Barbora; Indrajith, Suvasthika; Fárník, Michal; Fedor, Juraj (2018). Electron Attachment to Microhydrated Deoxycytidine Monophosphate. The Journal of Physical Chemistry B, acs.jpcb.8b03033. doi:10.1021/acs.jpcb.8b03033
Alizadeh, E., Sanche, L. (2012). Precursors of Solvated Electrons in Radiobiological Physics and Chemistry. Chemical Reviews, 112 (11), 5578–5602. doi:10.1021/cr300063r
Yandell, M. A.; King, S. B.; Neumark, D. M. (2013). Time-Resolved Radiation Chemistry: Photoelectron Imaging of Transient Negative Ions of Nucleobases. Journal of the American Chemical Society, 135(6), 2128–2131. doi:10.1021/ja312414y
Martin, Frédéric; Burrow, Paul D.; Cai, Zhongli; Cloutier, Pierre; Hunting, Darel; Sanche, Léon (2004). DNA Strand Breaks Induced by 0–4 eV Electrons: The Role of Shape Resonances. Physical Review Letters, 93(6), 068101. doi:10.1103/PhysRevLett.93.068101
Kouass Sahbani, S., Sanche, L., Cloutier, P., Bass, A. D., Hunting, D. J. (2014). Loss of Cellular Transformation Efficiency Induced by DNA Irradiation with Low-Energy (10 eV) Electrons. The Journal of Physical Chemistry B, 118(46), 13123–13131. doi:10.1021/jp508170c
Khorsandgolchin, G., Sanche, L., Cloutier, P., Wagner, J. Richard, R. (2019). Strand breaks induced by very low energy electrons: Product analysis and mechanistic insight into the reaction with TpT. Journal of the American Chemical Society. doi:10.1021/jacs.9b03295
Ptasinska, S., Denifl, S., Scheier, P., Illenberger, E. and Märk, T.D. (2005), Bond- and Site-Selective Loss of H Atoms from Nucleobases by Very-Low-Energy Electrons (<3 eV)†. Angewandte Chemie International Edition, 44, 6941-6943. doi:10.1002/anie.200502040
Kumar, A., Sevilla, M. D.; Suhai, S. (2008). Microhydration of the Guanine−Cytosine (GC) Base Pair in the Neutral and Anionic Radical States: A Density Functional Study. The Journal of Physical Chemistry B, 112(16), 5189–5198. doi:10.1021/jp710957p
Desfrançois, C. (1996). Electron attachment to isolated nucleic acid bases. The Journal of Chemical Physics, 104(19), 7792. doi:10.1063/1.471484
Ma, J., Kumar, A., Muroya, Y., Yamashita, S., Sakurai, T., Denisov, S., Sevilla, M. D.; Adhikary, A., Seki, S., Mostafavi, M., (2019). Observation of dissociative quasi-free electron attachment to nucleoside via excited anion radical in solution. Nature Communications, 10(1), 102. doi:10.1038/s41467-018-08005-z
Ma, J., Wang, F., Denisov, S. A.; Adhikary, A., Mostafavi, M. (2017). Reactivity of prehydrated electrons toward nucleobases and nucleotides in aqueous solution. Science Advances, 3(12), e1701669. doi:10.1126/sciadv.1701669
Gu, J., Leszczynski, J., Schaefer, H. F. (2012). Interactions of Electrons with Bare and Hydrated Biomolecules: From Nucleic Acid Bases to DNA Segments. Chemical Reviews, 112(11), 5603–5640. doi:10.1021/cr3000219
Zhao, Y., Truhlar, D. G. (2008). The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Acc., 120, 215–41. doi:10.1007/s00214-007-0310-x
Petersson, G. A., Bennett, A., Tensfeldt, T. G., Al-Laham, M. A., Shirley, W. A., Mantzaris, J. (1998). A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row atoms. J. Chem. Phys., 89, 2193–218. doi:10.1063/1.455064
Petersson, G. A., Al-Laham, M. A. (1991). A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys., 94, 6081-90. doi:10.1063/1.460447
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

