EXTRACTION OF NON-FERROUS METALS FROM AQUEOUS AMMONIUM SOLUTIONS
DOI:
https://doi.org/10.15421/jchemtech.v32i3.309559Keywords:
liquid-phase extraction; extraction; extraction system; extract; re-extractionAbstract
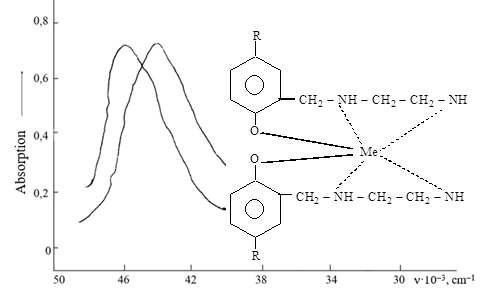
The work is devoted to the extraction of cadmium from ammonia aqueous solutions. The interaction of cadmium with 2-hydroxy-5-alkylbenzylethylenediamine in a wide pH range was studied. The composition of the extracted compounds was also determined. It has been shown that the state of cadmium in ammonia solutions depends on the pH value of the medium, as well as on the concentration of ammonium salt ions. It was found that cadmium in the reactions carried out is in amine forms – [Cd(NH3)4]2+. In this case, the extractant coordinates with the cadmium ion through the oxygen atoms of the phenolic OH group and the amine nitrogen group. This is confirmed by the fact that cadmium interacts with the reagent as a result of the exchange of the proton of the phenolic OH group and coordination by molecules of the NH group can be provided by the results of an IR spectroscopic study of the reagent and the extracted complex in CCl4, taken in the range of 700-4000 cm-1, characteristic of the appearance of ν-vibrations of hydroxyl and NH bonds in the molecules of the extracted compound. The IR spectra of the reagent and the extracted cadmium compound show that the characteristic absorption band of the OH group in the phenol molecule at a wavelength of 3590 cm-1 sharply decreases, which indicates the replacement of the hydroxyl group proton with the extracted metal ion and the formation of a Cd–O bond. In addition, the absorption band of the NH bond at 1640 cm-1 is shift into the short-wavelength region of the spectrum in the complex, which also indicates the participation of the NH group in the formation of the complex. These analyzes helped establish that indeed the cadmium ion in complex compounds is hydrogen bonded to molecules and forms a tetrahedral geometry.
References
Broeksma, C.P., Dorfling, C. (2023). Evaluating glycine as an alternative lixiviant for copper recovery from waste printed circuit boards, Waste Management, 163, 96–107; https://doi.org/10.1016/j.wasman.2023.03.030.
Schlesinger, M. E., Sole, K. C., Davenport, W. G., Gerardo, R.F., Alvear, F. (2021). Extractive Metallurgy of Copper. https://www.sciencedirect.com/book/9780128218754/extractive-metallurgy-of-copper
Srecko, S., Bernd, F. (2022). Advances in Understanding of Unit Operations in Non-ferrous Extractive Metallurgy 2021.
Gupta, B., Deep, A., Singh, V., Tandon, S.N. (2003). Recovery of cobalt, nickel and copper from sea nodules by their extraction with alkylphosphines. Hydrometallurgy. 70, 5121–129.
Chagnes, A. (2020). Advances in Hydrometallurgy. ISBN978-3-03928-939-4 . https://doi.org/10.3390/books978-3-0365-4574-5
Wang, Sh., Wang, X., Yang, J. (2022). Chemical Engineering and Technology in Mineral Processing and Extractive Metallurgy. https://doi.org/10.3390/books978-3-03928-940-0
Shekiliev, F.I. (2009). Extraction of copper with a mixture of naphthenic acids and bis-2-hydroxy-5-alkylbenzylamine from ammonia solutions Azerbaijan chemical journal. 2, 126–130.
Shekiliev, F.I. (2012). Study of the process of zinc extraction from ammonia solutions Journal of Actual Problems of the Humanities and Natural Sciences, 39(4), 20–24.
Shekiliev, F.I. (2013). Extraction of copper, cobalt and iron from ammonia solutions Journal of Natural and TechnicalSciences, 63(1), 55–58.
Shekiliev, F.I. (2010). Interaction of cobalt with a synergistic mixture of naphthenic acid and bis-2-hydroxy-5-alkylbenzylamine in extraction systems, Journal of Chemistry and Chemical Technology, 4, 33–37.
Degtev, M.I., Alikina, E.N. (2013). Extraction of Zn and Cd ions from thiocyanate solution swith melt mixtures of diantipyrylmethane and benzoic acid. Physical, mathematical and chemical sciences: theoretical trends and applied studies / education as the basis of the human evolution in conditions of the information environment of the society domination: materials digest of the LI International Research and Practice Conference and I stage of the Championship in physical, mathematical and chemical sciences, 11–14.
Degtev, M.I., Stankova, A.V. (2019). Assessment of the extraction ability of aqueous stratifying systems using the example of extraction of cadmium (II) and zinc (II) ions from hydrochloric acid solutions. News of hig here ducational institutions. Series: Chemistry and chemical technology, 62(11), 57–62.
Temerev, S.V., Petukhov, V.A. (2019). Determination of copper (II), cadmium (II), lead (II), zinc (II) ions in snow and water samples after extraction with thiopyrinium salicylate. Chemistry for sustainable development,, 27(4), 403–411.
Hassan, A. H., Abdul, W. M. (2023). Recovery of cadmium from aqueous ammonium solutions using solvent extraction techniques. Journal of Environmental Chemical Engineering, 11(3), 108157.
Liu, H., Wang, Sh., Fu, L., Zhang, G., Zuo, Y., Zhang, L. (2022). Mechanism and kinetics analysis of valuable metals leaching from copper-cadmium slag assisted by ultrasound cavitation, Journal of Cleaner Production, 379(2), 134775.
https://doi.org/10.1016/j.jclepro.2022.134775.
Zhang, D., He, F., Miao, Zh., Zhang, Ya D. (2021). Intensified extraction and separation of zinc from cadmium and manganese by a slug flow capillary microreactor. Separation and Purification Technology, 267, 118564.
https://doi.org/10.1016/j.seppur.2021.118564.
Kuimova, Zh. Yu., Alikina, E. N. (2023). Extraction of cadmium in the separating system diphenylguanidine–benzoic acid–water. Bulletin of the Perm University, ser. chemistry, 13(4), 213–223.
Rayco, L., Koen, B. (2021). Hard–Soft Interactions in Solvent Extraction with Basic Extractants: Comparing Zinc and Cadmium Halides. ACS Omega. 6(42), 27924–27935. doi: 10.1021/acsomega.1c03790
Charlot, G. (1965). Methods of analytical chemistry. Quantitative analysis of inorganic compounds. Moscow-Leningrad: Chemistry.
Guo, Sh., Zhang, F., Li, D., Jiao P. (2020). Highly efficient and selective removal of cadmium from aqueous solutions based on magnetic graphitic carbon nitride materials with molecularly imprinted polymers, Journal of Molecular Structure, 1221, 128887, https://doi.org/10.1016/j.molstruc.2020.128887.
Ehsan, B., Mehdi, I., Mahdi, G. (2013). Solvent extraction recovery and separation of cadmium and copper from sulphate solution, Journal of Environmental Chemical Engineering, 1(4), 1269–1274; https://doi.org/10.1016/j.jece.2013.09.016.
Rybak, B.M. (1964). Analysis of oil and petroleum products. M.: Gostoptekhizdat.
Nakamoto, K. (1991). IR and Raman spectra of inorganic and coordination compounds. Moscow: Mir. 536.
Bellamy, L. (1971). New data on the IR spectra of complex molecules. Moscow: Mir.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

