THEORETICAL STUDY OF THERMAL CURTIUS REARRANGEMENT MECHANISM FOR SOME ARYL- AS WELL AS HETARYLACYL AZIDES WITH PREDICTION OF THE FOLLOWING CYCLOTRIMERIZATION PROGRESS
DOI:
https://doi.org/10.15421/jchemtech.v32i4.310213Keywords:
ab initio розрахунки; термічне перегрупування Курціуса; енергія активації; метод поляризуючого континууму; механізм циклотримеризації.Abstract
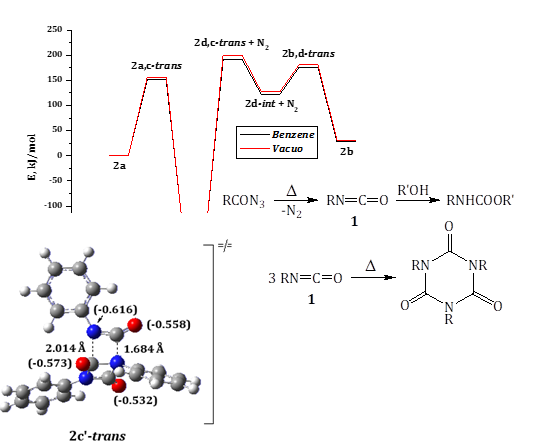
With the aid of ab initio density functional method in the non-empirical generalized gradient approximation on the example of syn-conformers of aryl- as well as hetarylacyl azides, which turned out to be thermodynamically more stable than the corresponding anti-analogues, some areas of the potential energy surfaces have been investigated for the thermal Curtius rearrangement, which occures according to a concerted mechanism and includes the cleavage of a nitrogen molecule under the simultaneous migration of an aromatic fragment with the corresponding isocyanate formation in vacuo as well as in bulk of benzene solution. The analysis of the calculated values of activation barriers showed that the introduction of a pyridine-type Nitrogen atom into the meta- and para-positions of the benzene ring practically does not affect on the EACT absolute values, while in the ortho-position such a modification of the substrate leads to only a slight decrease of it, especially in comparison with unsubstituted prototype. As for the non-catalytic cyclotrimerization processes, that can take place with the participation of obtained in the previous stage isocyanates, the only transition state corresponding to the concerted mechanism of this transformation has not been localized at all on the potential energy surface. Instead of the process takes place as stepwise and includes the sequential addition of an aryl isocyanate molecule to the dimer formed at the previous stage with the expansion of a four-membered ring to a six-membered one. The results of calculations are in good agreement with theoretical data, which have been obtained for such type modeling previously and indicating the low sensitivity of the reaction to the solvation effects of medium.
References
Ghosh, A. K., Sarkar, A., Brindisi, M. (2018). The Curtius rearrangement: Mechanistic insight and recent applications in natural product syntheses. Org. Biomol. Chem., 16, 2006–2027. doi: 10.1039/C8OB00138C
Wu, Z., Zeng, X. (2022). Curtius-Type Rearrangement of Sulfinyl Azides: A Matrix Isolation and Computational Study. J. Phys. Chem. A., 126(27), 4367–4375. doi: 10.1021/acs.jpca.2c02469
McCulla, R. D., Gohar, G. A., Hadad, C. M., Platz, M. S. (2007). Computational Study of the Curtius-like Rearrangements of Phosphoryl, Phosphinyl, and Phosphinoyl Azides and Their Corresponding Nitrenes. J. Org. Chem., 72(25), 9426–9438. doi: 10.1021/jo0711687
Tokar, A., Chihvintseva, O., Mirjanić, D. (2024). The Quantum-Chemical Aspects of Structuring for Some Aramide-Type Polymer Systems with Hetaryl Fragments. In: Karabegovic, I., Kovačević, A., Mandzuka, S. (eds.) New Technologies, Development and Application VII. NT 2024. Lecture Notes in Networks and Systems, 1070, 589–596. Springer, Cham. doi: 10.1007/978-3-031-66271-3_63
Tokar, A., Chigvintseva, O. (2021). The quantum-chemical and spectral criteria for hydrogen bonding efficiency in structural analysis of aramides. Chem. Chem. Technol., 15(1), 9–15. doi: 10.23939/chcht15.01.009
Guo, Y., Muuronen, M., Lucas, F., Sijbesma, R. P., Tomović, Ž. (2023). Catalysts for Isocyanate Cyclotrimerization. ChemCatChem, 15(10), e202201362. doi: 10.1002/cctc.202201362
Li, Ch., Zhao, W., He, J., Zhang, Y. (2019). Highly efficient cyclotrimerization of isocyanates by N-Heterocyclic Olefins under bulk condition. Chem. Commun., 55, 12563–12566. doi: 10.1039/C9CC06402H
Wolf, M. E., Vandezande, J. E., Schaefer, H. F. (2021). Catalyzed Reaction of Isocyanates (RNCO) with Water. Phys. Chem. Chem. Phys., 23, 18535–18546. doi: 10.1039/D1CP03302F
Ruipérez, F. (2019). Application of quantum chemical methods in polymer chemistry. Int. Rev. Phys. Chem., 38(3–4), 343–403. doi: 10.1080/0144235X.2019.1677062
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, Jr., J. A., Vreven, T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., Pople, J. A. (2004). Gaussian 03 (Revision E.01). Gaussian Inc., Wallingford CT.
Merrick, J. P., Moran, D., Radom, L. (2007). An Evaluation of Harmonic Vibrational Frequency Scale Factors. J. Phys. Chem. A., 111(45), 11683–11700. doi:
1021/jp073974n
Tomasi, J. (2011). Selected features of the polarizable continuum model for the representation of solvation. WIREs Comput. Mol. Sci., 1(5), 855–867. doi: 10.1002/wcms.54
Glendening, E. D., Hiatt, D. M., Weinhold, F. (2024). Natural Bond Orbital Analysis of Chemical Structure, Spectroscopy, and Reactivity: How it Works. Comprehensive Comput. Chem., 2, 406–421. doi: 10.1016/B978-0-12-821978-2.00077-5
Taherian, R., Chahkandi, B., Zahedi, E. (2021). A comprehensive theoretical analysis of Curtius rearrangement of syn-syn and syn-anti conformers of oxalyl diazide. J. Mol. Graphics Modell., 109, 108012. doi: 10.1016/j.jmgm.2021.108012
Godara, S., Radhakrishnan, A., Paranjothy, M. (2020). Chemical Dynamics Simulations of Curtius Reaction of Acetyl- and Fluorocarbonyl Azides. J. Phys. Chem. A., 124(32), 6438–6444. doi: 10.1021/acs.jpca.0c04366
Nouri, A., Zahedi, E., Ehsani, M., Nouri, A., Balali, E. (2018). Understanding the kinetics and molecular mechanism of the Curtius rearrangement of 3-oxocyclobutane-1-carbonyl azide. Comput. Theor. Chem., 1130, 121–129. doi: 10.1016/j.comptc.2018.03.019
Kakkar, R., Zaidi, S., Grover, R. (2009). The Curtius Rearrangement of Some Organic Azides: A DFT Mechanistic Study. Int. J. Quantum Chem., 109(5), 1058–1069. doi: 10.1002/qua.21911
Kishi, V., Chahkandi, B., Zahedi, E., Allameh, S. (2024). A theoretical assessment of Curtius rearrangement of malonyl azide: Molecular mechanism insight and solvent effects. J. Mol. Liq., 396, 124078. doi: 10.1016/j.molliq.2024.124078
Peng, X.-L., Ding, W.-L., Li, Q.-S., Li, Z.-S. (2017). Theoretical Insights into Photo-Induced Curtius Rearrangement of Chlorodifluoroacetyl Azide. Org. Chem. Front., 4, 1153–1161. doi: 10.1039/C7QO00083A
Xie, B.-B., Cui, Ch.-X., Fang, W.-H., Cui, G. (2018). Photoinduced Curtius rearrangements of fluorocarbonyl azide, FC(O)N3: a QM/MM nonadiabatic dynamics simulation. Phys. Chem. Chem. Phys., 20, 19363–19372. doi: 10.1039/C8CP02651C
Abu-Eittah, R. H., Hassan, W. M. I., Zordok, W. (2015). A theoretical study of the thermal Curtius rearrangement of some cinnamoyl azides using the DFT approach. J. Struct. Chem., 56(4), 628–641. doi: 10.1134/S0022476615040046
Tarwade, V., Dmitrenko, O., Bach, R. D., Fox, J. M. (2008). The Curtius Rearrangement of Cyclopropyl and Cyclopropenoyl Azides. A Combined Theoretical and Experimental Mechanistic Study. J. Org. Chem., 73(21), 8189–8197. doi: 10.1021/jo801104t
Williams, A., Williams, J. (2003). Free Energy Relationships in Organic and Bio-Organic Chemistry. Cambridge, UK: Royal Society of Chemistry.
Anslyn, E. V., Dougherty, D. A. (2005). Modern Physical Organic Chemistry. Sausalito, USA: University Science.
Wu, X., Mason, J., North, M. (2017). Isocyanurate Formation During Oxazolidinone Synthesis from Epoxides and Isocyanates Catalysed by a Chromium(Salphen) Complex. Chem. Eur. J., 23(52), 12937–12943. doi: 10.1002/chem.201702948
Tokar, A. V. (2014). The quantum-chemical investigation of N-cyclization reaction mechanism for epichlorohydrin aminolysis products. Visn. Dnipropetr. Univ.: Khim. – Bull. Dnipropetr. Univ.: Chem., 22(2), 27–30. doi: 10.15421/081418
Tokar, A. V., Petrushyna, H. О. (2018). [The quantum-chemical investigation of heterocyclization mechanism for oligomeric product of epichlorohydrin aminolysis: epoxide or the dioxane?]. J. Chem. Technol., 26(2), 12–19 (in Ukrainian). doi: 10.15421/0817260202
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

