STUDY ON TNT TOXIC EFFECTS ON THE FUNCTIONAL STATE OF HYDROBIONTS IN THE MODEL CONTAMINATED WATER POND
DOI:
https://doi.org/10.15421/jchemtech.v32i3.310257Keywords:
TNT, aquatic organisms, toxicity, GST, POL, erythrocytes pathologiesAbstract
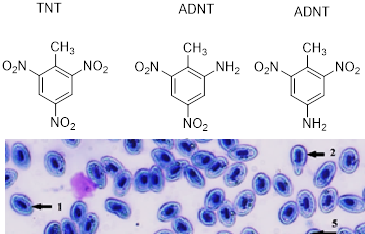
TNT (2,4,6-trinitrotoluene) and its derivatives make a significant contribution to the pollution of the water environment caused by hostilities in Ukraine, but the effects of TNT on hydrobionts have been little studied. In a model experiment, the effect of TNT in concentrations of 10 –75 mg/L on the mortality rate of Daphnia magna crustaceans was observed during 96 hours, and LD50 = 41.8 mg/L was calculated. Impact of TNT on Carassius gibelio fish metabolic processes and red blood cells characteristics were studied during chronic (21 days at a dose of 5 mg/L) and acute (eight hours at a dose of 35 mg/L) experiments. A sharp increase in lipid peroxidation and glutathione S-transferase activity were recorded in the blood and spleen of Carassius gibelio during the chronic TNT action indicating the state of power oxidative stress and detoxification mechanisms activation. Both chronic and acute exposure to TNT caused an increase in the destructive changes in red blood cells of Carassius gibelio as compared to control, including size and shape variation, decreased hemoglobin content, nuclear shift, and cell division disruption. Cytoplasmic vacuolization in fish erythrocytes was detected under the influence of both TNT concentrations, while karyolysis only under acute exposure. The obtained data testify to the severe pathological consequences of 2,4,6-trinitrotoluene impact on the viability and functional state of aquatic organisms under the model conditions and dictate the need to conduct similar studies in the natural polluted aquatic ecosystems to identify ways of their restoration.
References
Schillinger, J., Özerol, G., Güven‐Griemert, Ş., Heldeweg, M. (2020). Water in war: Understanding the impacts of armed conflict on water resources and their management. WIREs Water. 7(2), e1480. https://doi.org/10.1002/wat2.1480
Sharamok, T., Ananieva, T., Fedonenko, O. (2017). Environmental status of Kam’yanske reservoir (Ukraine). Ekológia (Bratislava), 36(3), 281–289. https://doi.org/10.1515/eko-2017-0023
[Digest of the key consequences of Russian aggression for the Ukrainian environment for July 7-13, 2022: the official website of the Ministry of Environmental Protection and Natural Resources] (in Ukrainian). https://mepr.gov.ua/news/39409.html.
POST-DISASTER NEEDS ASSESSMENT (PDNA). October 2023. Government of Ukraine and the United Nations. https://ukraine.un.org/sites/default/files/2023-10/PDNA%20Final%20and%20Cleared%20-%2016Oct.pdf
Alpatova, О., Maksymenko, I., Patseva, I., Khomiak, I., Gandziura, V. (2022). Hydrochemical state of the post-military operations water ecosystems of the Moschun, Kyiv region. 16th Int. Conf. Monitoring of Geological Processes and Ecological Condition of the Environment, 1–5. https://doi.org/10.3997/2214-4609.2022580145
Tsyhanenko-Dziubenko, I., Kireitseva, H., Demchuk, L. (2023). Dynamics of Heavy Metal Compounds Allocation in Urbohydrotops of Kyiv Region in Post-Military Conditions. 17th Int. Conf. Monitoring of Geological Processes and Ecological Condition of the Environment, 1–5. https://doi.org/10.3997/2214-4609.2023520066
Maser, E., Andresen, K., Bünning, T., Clausen, O., Wichert, U., Strehse, J. (2023). Ecotoxicological Risk of World War Relic Munitions in the Sea after Low- and High-Order Blast-in-Place Operations. Environ. Sci. Technol. 57(48), 20169–20181. https://doi.org/10.1021/acs.est.3c04873
Bergmann, S., Brenner, M., Strehse, J., Büning, T., Maser, E., Grassel, P., Heuskin, D., Brandt, D., Berger, M., van der Wulp, S., Skellhorn, M., Hill, P., Van Haelst, S., De Rijcke, M., Wichert, U. (2024). Risk assessment of war wrecks - a comprehensive approach investigating four wrecks containing munitions in the German Bight/North Sea. Propellants, Explos., Pyrotech,. 49, e202300322. https://doi.org/10.1002/prep.202300322
Beck, A., Gledhill, M., Kampmeier, M., Feng, C., Schlosser, C., Greinert, J., Achterberg, E. (2022). Explosives compounds from sea-dumped relic munitions accumulate in marine biota. Sci. Total Environ., 806(Pt 4), 151266. https://doi.org/10.1016/j.scitotenv.2021.151266
Maser, E., Bünning, T., Strehse, J. (2024). Environmental and human toxicology studies on explosive chemicals leaking from submerged munitions. Propell Explos Pyrot., 49(4), e202300181. https://doi.org/10.1002/prep.202300181
Esteve-Núñez, A., Caballero, A., Ramos, J.L. (2001). Biological degradation of 2,4,6-trinitrotoluene. Microbiol Mol Biol Rev., 65(3), 335–352. https://doi.org/10.1128/mmbr.65.3.335-352.2001
Lynch, J.C., Myers, K.F., Brannon, J.M., Delfino, J.J. (2001). Effects of pH and temperature on the aqueous solubility and dissolution rate of 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-l,3,5-triazine (RDX), and octahydro-1,3,5,7-tetranitro-l,3,5,7-tetrazocine (HMX). JCED, 46(6), 1549–1555. http://dx.doi.org/10.1021/je0101496
Xu, M., He, L., Sun, P., Wu, M., Cui, X., Liu, D., Adomako-Bonsu, A.G., Geng, M., Xiong, G., Guo, L., Maser, M. (2023). Critical Role of Monooxygenase in Biodegradation of 2,4,6-Trinitrotoluene by Buttiauxella sp. S19-1. Molecules, 28(4), 1969. https://doi.org/10.3390/molecules28041969
Rosen, G., Lotufo, G., Belden, J., George, R. (2022). Environmental Characterization of Underwater Munitions Constituents at a Former Military Training Range. Environ. Toxicol. Chem., 41(2), 275–286. https://doi.org/10.1002/etc.5112
Scharsack, J., Koske, D., Straumer, K., Kammann, U. (2021). Effects of climate change on marine dumped munitions and possible consequence for inhabiting biota. Environ Sci Eur., 33, 102. https://doi.org/10.1186/s12302-021-00537-4
Aamir, M., Irum, S., Siddiq, A., Batool, H., Ahmed, N., Awais, M., Ali, S. (2022). A novel method development and validation for determination of 2,4,6-Trinitrotoluene and its metabolites on LC-MS/MS. Anal. Biochem., 638, 114496. https://doi.org/10.1016/j.ab.2021.114496
Bünning, T., Strehse, J., Hollmann, A., Bötticher, T., Maser, E. (2021). A Toolbox for the Determination of Nitroaromatic Explosives in Marine Water, Sediment, and Biota Samples on Femtogram Levels by GC-MS/MS. Toxics, 9(3), 60. https://doi.org/10.3390/toxics9030060
Mishra, S., Marutheeswaran, S., Roy, S., Natarajan, V., Rai, P., Nanda, B. (2021). Adsorption and degradation mechanism of 2,4,6-trinitrotoluene on TiO2 (110) surface. Surf. Sci., 713. 121902. https://doi.org/10.1016/j.susc.2021.121902
Nguyen, H., Pham, S., Vu, T., Nguyen, H., La, D. (2024). Effective treatment of 2,4,6-trinitrotoluene from aqueous media using a sono-photo-Fenton-like process with a zero-valent iron nanoparticle (nZVI) catalyst. RSC Adv., 14, 23720–23729. https://doi.org/10.1039/d4ra03907f
Yang, H., Zhou, M., Li, H., Liu, L., Zhou, Y., Long, X. (2019). Collective absorption of 2,4,6-trinitrotoluene into lipid membranes and its effects on bilayer properties. A computational study. RSC Advances, 9(67), 39046–39054. https://doi.org/10.1039/c9ra08408h
Khromykh, N.O., Marenkov, O.M., Sharamok, T.S., Anishchenko, A.O., Yesipova, N. B., Nesterenko, O. S., Kurchenko, V. O., Mylostyvyi, R. V. (2023). Simulating TNT (2,4,6-trinitrotoluene) elimination in the water pond inhabited by freshwater alga of the Rhizoclonium genus. Regulatory Mechanisms in Biosystems, 14(3), 365–369. https://doi.org/10.15421/10.15421/022354
Ownby, D.R., Belden, J.B., Lotufo, G.R., Lydy, M.J. (2005). Accumulation of trinitrotoluene (TNT) in aquatic organisms: part 1--Bioconcentration and distribution in channel catfish (Ictalurus punctatus). Chemosphere, 58(9), 1153–1159. https://doi.org/10.1016/j.chemosphere.2004.09.059
Ek, H., Dave, G., Sturve, J., Almroth, B.C., Stephensen, E., Förlin, L., Birgersson, G. (2005). Tentative biomarkers for 2,4,6-trinitrotoluene (TNT) in fish (Oncorhynchus mykiss). Aquat Toxicol., 72(3), 221–230. https://doi.org/10.1016/j.aquatox.2005.01.001
Della Torre, C., Corsi, I., Arukwe, A., Alcaro, L., Amato, E., Focardi, S. (2008). Effects of 2,4,6-trinitrotoluene (TNT) on phase I and phase II biotransformation enzymes in European eel Anguilla anguilla (Linnaeus, 1758). Marine Environmental Research, 66(1), 9–11. http://dx.doi.org/10.1016/j.marenvres.2008.02.008
Lin, H., Lin, F., Yuan, J., Cui, F., Chen, J. (2021). Toxic effects and potential mechanisms of Fluxapyroxad to zebrafish (Danio rerio) embryos. Sci. Total Environ., 769, 144519. https://doi.org/10.1016/j.scitotenv.2020.144519
Witeska, M., Kondera, E., Bojarski, B. (2023). Hematological and Hematopoietic Analysis in Fish Toxicology – A Review. Animals, 13(16), 2625. https://doi.org/10.3390/ani13162625
Martinez-Alvarez, R. M., Morales, A. E., Sanz, A. (2005). Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fisheries, 15(1), 75–88. https://doi.org/10.1007/s11160-005-7846-4
Shi, F., Yao, M., Huang, Y., Chen, Z., Xiao, J., Zhan, F., Li, Y., Lin, L., Qin, Z. (2023). Effects of antibiotics on immunity and apoptosis on grass carp liver and hepatocytes. J. Environ. Chem. Eng., 11(3), 110168. https://doi.org/10.1016/j.jece.2023.110168
Gaschler, M.M., Stockwell, B.R. (2017). Lipid peroxidation in cell death. BBRC, 482(3), 419–425. https://doi.org/10.1016/j.bbrc.2016.10.086
Tsikas, D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem., 524, 13–30. https://doi.org/10.1016/j.ab.2016.10.021
Mas-Bargues, C., Escrivá, C., Dromant, M., Borrás, C., Viña, J. (2021). Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. ABB, 709, 108941. https://doi.org/10.1016/j.abb.2021.108941
Garcia, D., Lima, D., da Silva, D.G.H., de Almeida, E.A. (2020). Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: Evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water. Ecotoxicol. Environ. Saf., 190, 110107. https://doi.org/10.1016/j.ecoenv.2019.110107
Hamed, R.R., Saleh, N.S., Shokeer, A., Guneidy, R.A., Abdel-Ghany, S.S. (2016). Glutathione and its related enzymes in the gonad of Nile Tilapia (Oreochromis niloticus). Fish Physiol Biochem., 2(1), 353–363. https://doi.org/10.1007/s10695-015-0143-9
Della Torre, C., Corsi, I., Arukwe, A., Valoti, M., Focardi, S. (2008). Interactions of 2,4,6-trinitrotoluene (TNT) with xenobiotic biotransformation system in European eel Anguilla anguilla (Linnaeus, 1758). Ecotoxicol. Environ. Saf., 71(3), 798–805. https://doi.org/10.1016/j.ecoenv.2008.03.003
Jenkins, T.F., Walsh, M.E. (1992). Development of field screening methods for TNT, 2,4-DNT and RDX in soil. Talanta, 39(4), 419–428. https://doi.org/10.1016/0039-9140(92)80158-A
Arsan, O.M., Davydov, O.A., Dyachenko, T.M., Yevtushenko, M.Y., Zhukynski, V.M., Kyrpenko, N.I., Yakushyn, V.M. (2006). [Methods of hydro ecological research of surface waters]. Ed. by Romanenko, V.D. Kyiv: Logos, 340 – 359 (in Ukrainian).
Kröger, M., Fels, G. (2000). 14C-TNT synthesis revisited. Journal of Labelled Compounds and Radiophar-maceuticals, 43(3), 217–227. https://doi.org/10.1002/(SICI)1099-1344(20000315)43:3%3C217::AID-JLCR305%3E3.0.CO;2-U
Кurchenko, V., Sharamok, T. (2020). The Hematological Parameters of the Prussian Carp (Carassius gibelio Bloch, 1782) Under the Zaporizhian (Dnipro) Reservoir Conditions. Turk. J. Fish. & Aquat. Sci., 20(11), 807–812. http://doi.org/10.4194/1303-2712-v20_11_04
Davydov, O.N., Temnikhanov, Y. D., Kurovska, L.Y. (2006). [Pathology of fish blood]. Kyiv: Ukrainski fitositsiologicheski tsentr, 46–47 (in Ukrainian).
Habig, W.H., Jakoby, W.B. (1981). Assays for differentiation of glutathione S-transferases. Meth Enzymol., 77, 398–405. https://doi.org/10.1016/s0076-6879(81)77053-8
Regulations on the Committee on Ethics (Bioethics): Regulatory document of the Ministry of Education, Science, Youth and Sports of Ukraine. Order dated 19.11.2012 No. 1287). http://www.mon.gov.ua/ua/activity/63/64/normativno-pravova-baza/
Hansson, T., Schiedek, D., Lehtonen, K.K., Vuorinen, P.J., Liewenborg, Noaksson, E., Tjarnlund, U., Hansson, M., Balk L. (2006). Biochemical biomarkers in adult female perch (Perca fluviatilis) in a chronically polluted gradient in the Stockholm recipient (Sweden). Mar. Pollut. Bull., 53(8-9), 451–468. https://doi.org/10.1016/j.marpolbul.2006.04.014
Marenkov, O. M., Izhboldina, O. O., Nazarenko, M. M., Mylostyvyi, R. V., Khramkova, O. M., Kapshuk, N. O., Prychepa M. V., Nesterenko, O. S. (2021). Influence of heavy metals on physiological and biochemical parameters of Pseudorasbora parva (Cypriniformes, Cyprinidae). Regul Mech Biosyst., 12(4), 745–752. https://doi.org/10.15421/0221103
Dias de Moraes, F., Perri Venturini, F., Rossi, P.A., Marchioni Avilez, I., da Silva de Souza, N.E., Moraes, G. (2018). Assessment of biomarkers in the neotropical fish Brycon amazonicus exposed to cypermethrin based insecticide. Ecotoxicology, 27, 188–197. https://doi.org/10.1007/s10646-017-1884-2
Kaur, K., Kaur, A. (2015). Fish erythrocytes as biomarkers for the toxicity of sublethal doses of an azo dye, Basic violet-1 (CI: 42535). Microscopy and Microanalysis, 21(1), 264–273. https://doi.org/10.1017/S1431927614013609
Pomortseva, N. A., Rodionova, N. K., Gudkov, D. I., Kaglyan O. Y. (2024). Quantitative and Qualitative Composition of the Peripheral Blood of Fish in the Gradient of Long-Term Radiation Exposure. Hydrobiological Journal, 60(1), 84–100. https://doi.org/10.1615/HydrobJ.v60.i1.60
Ahmed, I., Reshi, Q.M., Fazio, F. (2022). The influence of the endogenous and exogenous factors on hematological parameters in different fish species: a review. Aquacult Int., 28, 869–899. https://doi.org/10.1007/s10499-019-00501-3.
Bardhan, A., Abraham, T.J., Das, R., Patil, P. (2024). Visualization of poikilocytosis as an emerging erythrocytic biomarker for fish health assessment. AROH, 2(2), 136–157. https://doi.org/10.1002/aro2.47
Shahjahan, Md., Rahman, M., Islam, S. M. M., Uddin, Md. H., Al-Emran, Md. (2019). Increase in water temperature increases acute toxicity of sumithion causing nuclear and cellular abnormalities in peripheral erythrocytes of zebrafish Danio rerio. Environ Sci Pollut Res Int., 26(36), 36903–36912. https://doi.org/10.1007/s11356-019-06886-1
Fazio, F. (2019). Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture, 500, 237–242. https://doi.org/10.1016/j.aquaculture.2018.10.030
Javed, M., Ahmad, I., Ahmad, A., Usmani, N., Ahmad, M. (2016). Studies on the alterations in hematological indices, micronuclei induction and pathological marker enzyme activities in Channa punctatus (spotted snakehead) perciformes, channidae exposed to thermal power plant effluent. SpringerPlus, 5, 761. https://doi.org/10.1186/s40064-016-2478-9
Omar, W.A., Zaghloul, K.H., Abdel-Khalek, A.A., Abo-Hegab, S. (2012). Genotoxic effects of metal pollution in two fish species, Oreochromis niloticus and Mugil cephalus, from highly degraded aquatic habitats. MRGTEM, 746(1), 7–14. https://doi.org/10.1016/j.mrgentox.2012.01.013
Pomortseva, N. A., Gudkov, D. I. (2019). Effect of additional acute irradiation on cytomorphological abnormalities of erythrocytes of the Prussian carp (Carassius gibelio Bloch) from water body contaminated with radionuclides. Problems of Radiation Medicine & Radiobiology, 24, 270–283. http://dx.doi.org/10.33145/2304-8336-2019-24-270-283
Nemkov, T., Qadri, S. M., Sheffield, W. P., D’Alessandro, A. (2020). Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells. Blood Transfus., 18(2), 130–142. https://doi.org/10.2450/2020.0256-19
Schillinger, J., Özerol, G., Güven‐Griemert, Ş., Heldeweg, M. (2020). Water in war: Understanding the impacts of armed conflict on water resources and their management. WIREs Water. 7(2), e1480. https://doi.org/10.1002/wat2.1480
Sharamok, T., Ananieva, T., Fedonenko, O. (2017). Environmental status of Kam’yanske reservoir (Ukraine). Ekológia (Bratislava), 36(3), 281–289. https://doi.org/10.1515/eko-2017-0023
[Digest of the key consequences of Russian aggression for the Ukrainian environment for July 7-13, 2022: the official website of the Ministry of Environmental Protection and Natural Resources] (in Ukrainian). https://mepr.gov.ua/news/39409.html.
POST-DISASTER NEEDS ASSESSMENT (PDNA). October 2023. Government of Ukraine and the United Nations. https://ukraine.un.org/sites/default/files/2023-10/PDNA%20Final%20and%20Cleared%20-%2016Oct.pdf
Alpatova, О., Maksymenko, I., Patseva, I., Khomiak, I., Gandziura, V. (2022). Hydrochemical state of the post-military operations water ecosystems of the Moschun, Kyiv region. 16th Int. Conf. Monitoring of Geological Processes and Ecological Condition of the Environment, 1–5. https://doi.org/10.3997/2214-4609.2022580145
Tsyhanenko-Dziubenko, I., Kireitseva, H., Demchuk, L. (2023). Dynamics of Heavy Metal Compounds Allocation in Urbohydrotops of Kyiv Region in Post-Military Conditions. 17th Int. Conf. Monitoring of Geological Processes and Ecological Condition of the Environment, 1–5. https://doi.org/10.3997/2214-4609.2023520066
Maser, E., Andresen, K., Bünning, T., Clausen, O., Wichert, U., Strehse, J. (2023). Ecotoxicological Risk of World War Relic Munitions in the Sea after Low- and High-Order Blast-in-Place Operations. Environ. Sci. Technol. 57(48), 20169–20181. https://doi.org/10.1021/acs.est.3c04873
Bergmann, S., Brenner, M., Strehse, J., Büning, T., Maser, E., Grassel, P., Heuskin, D., Brandt, D., Berger, M., van der Wulp, S., Skellhorn, M., Hill, P., Van Haelst, S., De Rijcke, M., Wichert, U. (2024). Risk assessment of war wrecks - a comprehensive approach investigating four wrecks containing munitions in the German Bight/North Sea. Propellants, Explos., Pyrotech,. 49, e202300322. https://doi.org/10.1002/prep.202300322
Beck, A., Gledhill, M., Kampmeier, M., Feng, C., Schlosser, C., Greinert, J., Achterberg, E. (2022). Explosives compounds from sea-dumped relic munitions accumulate in marine biota. Sci. Total Environ., 806(Pt 4), 151266. https://doi.org/10.1016/j.scitotenv.2021.151266
Maser, E., Bünning, T., Strehse, J. (2024). Environmental and human toxicology studies on explosive chemicals leaking from submerged munitions. Propell Explos Pyrot., 49(4), e202300181. https://doi.org/10.1002/prep.202300181
Esteve-Núñez, A., Caballero, A., Ramos, J.L. (2001). Biological degradation of 2,4,6-trinitrotoluene. Microbiol Mol Biol Rev., 65(3), 335–352. https://doi.org/10.1128/mmbr.65.3.335-352.2001
Lynch, J.C., Myers, K.F., Brannon, J.M., Delfino, J.J. (2001). Effects of pH and temperature on the aqueous solubility and dissolution rate of 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-l,3,5-triazine (RDX), and octahydro-1,3,5,7-tetranitro-l,3,5,7-tetrazocine (HMX). JCED, 46(6), 1549–1555. http://dx.doi.org/10.1021/je0101496
Xu, M., He, L., Sun, P., Wu, M., Cui, X., Liu, D., Adomako-Bonsu, A.G., Geng, M., Xiong, G., Guo, L., Maser, M. (2023). Critical Role of Monooxygenase in Biodegradation of 2,4,6-Trinitrotoluene by Buttiauxella sp. S19-1. Molecules, 28(4), 1969. https://doi.org/10.3390/molecules28041969
Rosen, G., Lotufo, G., Belden, J., George, R. (2022). Environmental Characterization of Underwater Munitions Constituents at a Former Military Training Range. Environ. Toxicol. Chem., 41(2), 275–286. https://doi.org/10.1002/etc.5112
Scharsack, J., Koske, D., Straumer, K., Kammann, U. (2021). Effects of climate change on marine dumped munitions and possible consequence for inhabiting biota. Environ Sci Eur., 33, 102. https://doi.org/10.1186/s12302-021-00537-4
Aamir, M., Irum, S., Siddiq, A., Batool, H., Ahmed, N., Awais, M., Ali, S. (2022). A novel method development and validation for determination of 2,4,6-Trinitrotoluene and its metabolites on LC-MS/MS. Anal. Biochem., 638, 114496. https://doi.org/10.1016/j.ab.2021.114496
Bünning, T., Strehse, J., Hollmann, A., Bötticher, T., Maser, E. (2021). A Toolbox for the Determination of Nitroaromatic Explosives in Marine Water, Sediment, and Biota Samples on Femtogram Levels by GC-MS/MS. Toxics, 9(3), 60. https://doi.org/10.3390/toxics9030060
Mishra, S., Marutheeswaran, S., Roy, S., Natarajan, V., Rai, P., Nanda, B. (2021). Adsorption and degradation mechanism of 2,4,6-trinitrotoluene on TiO2 (110) surface. Surf. Sci., 713. 121902. https://doi.org/10.1016/j.susc.2021.121902
Nguyen, H., Pham, S., Vu, T., Nguyen, H., La, D. (2024). Effective treatment of 2,4,6-trinitrotoluene from aqueous media using a sono-photo-Fenton-like process with a zero-valent iron nanoparticle (nZVI) catalyst. RSC Adv., 14, 23720–23729. https://doi.org/10.1039/d4ra03907f
Yang, H., Zhou, M., Li, H., Liu, L., Zhou, Y., Long, X. (2019). Collective absorption of 2,4,6-trinitrotoluene into lipid membranes and its effects on bilayer properties. A computational study. RSC Advances, 9(67), 39046–39054. https://doi.org/10.1039/c9ra08408h
Khromykh, N.O., Marenkov, O.M., Sharamok, T.S., Anishchenko, A.O., Yesipova, N. B., Nesterenko, O. S., Kurchenko, V. O., Mylostyvyi, R. V. (2023). Simulating TNT (2,4,6-trinitrotoluene) elimination in the water pond inhabited by freshwater alga of the Rhizoclonium genus. Regulatory Mechanisms in Biosystems, 14(3), 365–369. https://doi.org/10.15421/10.15421/022354
Ownby, D.R., Belden, J.B., Lotufo, G.R., Lydy, M.J. (2005). Accumulation of trinitrotoluene (TNT) in aquatic organisms: part 1--Bioconcentration and distribution in channel catfish (Ictalurus punctatus). Chemosphere, 58(9), 1153–1159. https://doi.org/10.1016/j.chemosphere.2004.09.059
Ek, H., Dave, G., Sturve, J., Almroth, B.C., Stephensen, E., Förlin, L., Birgersson, G. (2005). Tentative biomarkers for 2,4,6-trinitrotoluene (TNT) in fish (Oncorhynchus mykiss). Aquat Toxicol., 72(3), 221–230. https://doi.org/10.1016/j.aquatox.2005.01.001
Della Torre, C., Corsi, I., Arukwe, A., Alcaro, L., Amato, E., Focardi, S. (2008). Effects of 2,4,6-trinitrotoluene (TNT) on phase I and phase II biotransformation enzymes in European eel Anguilla anguilla (Linnaeus, 1758). Marine Environmental Research, 66(1), 9–11. http://dx.doi.org/10.1016/j.marenvres.2008.02.008
Lin, H., Lin, F., Yuan, J., Cui, F., Chen, J. (2021). Toxic effects and potential mechanisms of Fluxapyroxad to zebrafish (Danio rerio) embryos. Sci. Total Environ., 769, 144519. https://doi.org/10.1016/j.scitotenv.2020.144519
Witeska, M., Kondera, E., Bojarski, B. (2023). Hematological and Hematopoietic Analysis in Fish Toxicology – A Review. Animals, 13(16), 2625. https://doi.org/10.3390/ani13162625
Martinez-Alvarez, R. M., Morales, A. E., Sanz, A. (2005). Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fisheries, 15(1), 75–88. https://doi.org/10.1007/s11160-005-7846-4
Shi, F., Yao, M., Huang, Y., Chen, Z., Xiao, J., Zhan, F., Li, Y., Lin, L., Qin, Z. (2023). Effects of antibiotics on immunity and apoptosis on grass carp liver and hepatocytes. J. Environ. Chem. Eng., 11(3), 110168. https://doi.org/10.1016/j.jece.2023.110168
Gaschler, M.M., Stockwell, B.R. (2017). Lipid peroxidation in cell death. BBRC, 482(3), 419–425. https://doi.org/10.1016/j.bbrc.2016.10.086
Tsikas, D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem., 524, 13–30. https://doi.org/10.1016/j.ab.2016.10.021
Mas-Bargues, C., Escrivá, C., Dromant, M., Borrás, C., Viña, J. (2021). Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. ABB, 709, 108941. https://doi.org/10.1016/j.abb.2021.108941
Garcia, D., Lima, D., da Silva, D.G.H., de Almeida, E.A. (2020). Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: Evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water. Ecotoxicol. Environ. Saf., 190, 110107. https://doi.org/10.1016/j.ecoenv.2019.110107
Hamed, R.R., Saleh, N.S., Shokeer, A., Guneidy, R.A., Abdel-Ghany, S.S. (2016). Glutathione and its related enzymes in the gonad of Nile Tilapia (Oreochromis niloticus). Fish Physiol Biochem., 2(1), 353–363. https://doi.org/10.1007/s10695-015-0143-9
Della Torre, C., Corsi, I., Arukwe, A., Valoti, M., Focardi, S. (2008). Interactions of 2,4,6-trinitrotoluene (TNT) with xenobiotic biotransformation system in European eel Anguilla anguilla (Linnaeus, 1758). Ecotoxicol. Environ. Saf., 71(3), 798–805. https://doi.org/10.1016/j.ecoenv.2008.03.003
Jenkins, T.F., Walsh, M.E. (1992). Development of field screening methods for TNT, 2,4-DNT and RDX in soil. Talanta, 39(4), 419–428. https://doi.org/10.1016/0039-9140(92)80158-A
Arsan, O.M., Davydov, O.A., Dyachenko, T.M., Yevtushenko, M.Y., Zhukynski, V.M., Kyrpenko, N.I., Yakushyn, V.M. (2006). [Methods of hydro ecological research of surface waters]. Ed. by Romanenko, V.D. Kyiv: Logos, 340 – 359 (in Ukrainian).
Kröger, M., Fels, G. (2000). 14C-TNT synthesis revisited. Journal of Labelled Compounds and Radiophar-maceuticals, 43(3), 217–227. https://doi.org/10.1002/(SICI)1099-1344(20000315)43:3%3C217::AID-JLCR305%3E3.0.CO;2-U
Кurchenko, V., Sharamok, T. (2020). The Hematological Parameters of the Prussian Carp (Carassius gibelio Bloch, 1782) Under the Zaporizhian (Dnipro) Reservoir Conditions. Turk. J. Fish. & Aquat. Sci., 20(11), 807–812. http://doi.org/10.4194/1303-2712-v20_11_04
Davydov, O.N., Temnikhanov, Y. D., Kurovska, L.Y. (2006). [Pathology of fish blood]. Kyiv: Ukrainski fitositsiologicheski tsentr, 46–47 (in Ukrainian).
Habig, W.H., Jakoby, W.B. (1981). Assays for differentiation of glutathione S-transferases. Meth Enzymol., 77, 398–405. https://doi.org/10.1016/s0076-6879(81)77053-8
Regulations on the Committee on Ethics (Bioethics): Regulatory document of the Ministry of Education, Science, Youth and Sports of Ukraine. Order dated 19.11.2012 No. 1287). http://www.mon.gov.ua/ua/activity/63/64/normativno-pravova-baza/
Hansson, T., Schiedek, D., Lehtonen, K.K., Vuorinen, P.J., Liewenborg, Noaksson, E., Tjarnlund, U., Hansson, M., Balk L. (2006). Biochemical biomarkers in adult female perch (Perca fluviatilis) in a chronically polluted gradient in the Stockholm recipient (Sweden). Mar. Pollut. Bull., 53(8-9), 451–468. https://doi.org/10.1016/j.marpolbul.2006.04.014
Marenkov, O. M., Izhboldina, O. O., Nazarenko, M. M., Mylostyvyi, R. V., Khramkova, O. M., Kapshuk, N. O., Prychepa M. V., Nesterenko, O. S. (2021). Influence of heavy metals on physiological and biochemical parameters of Pseudorasbora parva (Cypriniformes, Cyprinidae). Regul Mech Biosyst., 12(4), 745–752. https://doi.org/10.15421/0221103
Dias de Moraes, F., Perri Venturini, F., Rossi, P.A., Marchioni Avilez, I., da Silva de Souza, N.E., Moraes, G. (2018). Assessment of biomarkers in the neotropical fish Brycon amazonicus exposed to cypermethrin based insecticide. Ecotoxicology, 27, 188–197. https://doi.org/10.1007/s10646-017-1884-2
Kaur, K., Kaur, A. (2015). Fish erythrocytes as biomarkers for the toxicity of sublethal doses of an azo dye, Basic violet-1 (CI: 42535). Microscopy and Microanalysis, 21(1), 264–273. https://doi.org/10.1017/S1431927614013609
Pomortseva, N. A., Rodionova, N. K., Gudkov, D. I., Kaglyan O. Y. (2024). Quantitative and Qualitative Composition of the Peripheral Blood of Fish in the Gradient of Long-Term Radiation Exposure. Hydrobiological Journal, 60(1), 84–100. https://doi.org/10.1615/HydrobJ.v60.i1.60
Ahmed, I., Reshi, Q.M., Fazio, F. (2022). The influence of the endogenous and exogenous factors on hematological parameters in different fish species: a review. Aquacult Int., 28, 869–899. https://doi.org/10.1007/s10499-019-00501-3.
Bardhan, A., Abraham, T.J., Das, R., Patil, P. (2024). Visualization of poikilocytosis as an emerging erythrocytic biomarker for fish health assessment. AROH, 2(2), 136–157. https://doi.org/10.1002/aro2.47
Shahjahan, Md., Rahman, M., Islam, S. M. M., Uddin, Md. H., Al-Emran, Md. (2019). Increase in water temperature increases acute toxicity of sumithion causing nuclear and cellular abnormalities in peripheral erythrocytes of zebrafish Danio rerio. Environ Sci Pollut Res Int., 26(36), 36903–36912. https://doi.org/10.1007/s11356-019-06886-1
Fazio, F. (2019). Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture, 500, 237–242. https://doi.org/10.1016/j.aquaculture.2018.10.030
Javed, M., Ahmad, I., Ahmad, A., Usmani, N., Ahmad, M. (2016). Studies on the alterations in hematological indices, micronuclei induction and pathological marker enzyme activities in Channa punctatus (spotted snakehead) perciformes, channidae exposed to thermal power plant effluent. SpringerPlus, 5, 761. https://doi.org/10.1186/s40064-016-2478-9
Omar, W.A., Zaghloul, K.H., Abdel-Khalek, A.A., Abo-Hegab, S. (2012). Genotoxic effects of metal pollution in two fish species, Oreochromis niloticus and Mugil cephalus, from highly degraded aquatic habitats. MRGTEM, 746(1), 7–14. https://doi.org/10.1016/j.mrgentox.2012.01.013
Pomortseva, N. A., Gudkov, D. I. (2019). Effect of additional acute irradiation on cytomorphological abnormalities of erythrocytes of the Prussian carp (Carassius gibelio Bloch) from water body contaminated with radionuclides. Problems of Radiation Medicine & Radiobiology, 24, 270–283. http://dx.doi.org/10.33145/2304-8336-2019-24-270-283
Nemkov, T., Qadri, S. M., Sheffield, W. P., D’Alessandro, A. (2020). Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells. Blood Transfus., 18(2), 130–142. https://doi.org/10.2450/2020.0256-19
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

