SYNTHESIS AND CHARACTERIZATION OF SILICATE GLASS FROM ALTERNATIVE RAW MATERIALS USING HYDROTHERMAL CHARGE
DOI:
https://doi.org/10.15421/jchemtech.v33i1.311109Keywords:
glass, X-ray, thermal analysis, perlite, diatomite, hydrothermal batchAbstract
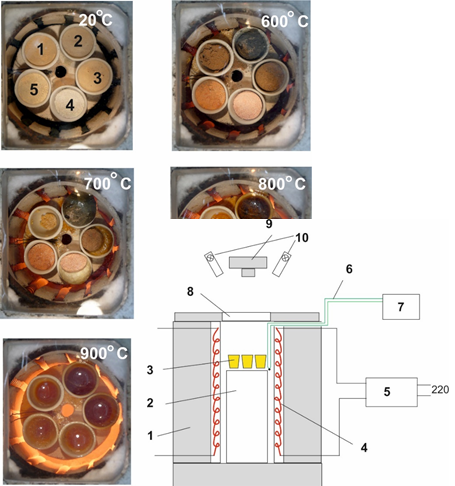
Aim. To study the possibility of expanding the raw material base of glass production due to the use of high-silica raw materials – diatomite and perlite. Thus, the introduction of the main component of silicate glass SiO2 occurs due to amorphous silica contained in rocks. Methods. The batch was prepared by hydrothermal method. Autoclave processing of rocks and silica was carried out for 4 hours at a temperature of 170 °С and a pressure of 0.5 MPa. NaOH was used as an alkaline agent. The molar ratio of SiO2 to NaOH was equal to 2. The SiO2–Na2O–PbO system and the glass composition in mole fractions were selected for the studies: SiO2 – 52.4 %; Na2O – 26.2 %; PbO – 21.4 %. The composition point lies on the liquidus isotherm of about 800 °C. The glass synthesis was carried out at a temperature of 1100 °C. Results. The study of the kinetics of glass making showed that when using hydrothermal making batches based on rocks, the stage of obtaining conditioned glass mass occurs at significantly lower temperatures than in the case of glass making from traditional batches and is at least 100 °С. Thus, it can be argued that the use of hydrothermal batches will reduce energy costs at the glass making stage. DTA results confirmed that the physicochemical activity of batches using amorphous silica is lower than for batches made using crystalline silica. X-ray confirmed that all glass samples welded at a temperature of 1000 °С are characterized by high X-ray amorphousness, which indicates the completion of glass formation processes. A visual assessment of the processes of silicate and glass formation showed that the boiling temperature of model glass based on hydrthermal batches is 110–120 °С lower than for glass made on the basis of traditional batches. Conclusions. Thus, it can be argued that the use of alternative raw materials, diatomite and perlite, will allow obtaining silicate glass. And the use of the hydrothermal method for obtaining the charge allows for a reduction in energy consumption during the glassmaking stage.
References
Reka, A. A., Durmishi, B., Jashari, A., Pavlovski, B., Buxhaku, N., Durmishi, A. (2016). Physical-chemical and mineralogical-petrographic examinations of tri poli from Republic of Macedonia. Int J Innov Stud Sci Eng Technol, 2(1), 13-7. https://doi.org/10.1515/chem-2019-0132.
Movsisyan, M.S., Gevorkyan, A.C., Grigoryan, O.V. (1989). Study of the processes of autoclave dissolution of quartz sand and high-siliceous rock - tripoli from the Fokinsky deposit. Armenian Chemical Journal. 42(2): 77–82.
Bagramyan, V.V., Sarkisyan, A.A., Ponzoni, C., Rosa, R., Leonelli, C. (2015). Microwaveassisted preparation of sodium-silicate solutions from perlite, Theor. Found. Chem. Eng. 49(5), 731–735, doi: 10.1134/S0040579515050048
Sharafudeen, R., Al-Hashim, J.M., Al-Harbi, M.O., Al-Ajwad, A.I., Al-Waheed, A.A. (2017). Preparation and characterization of precipitated silica using sodium silicate prepared from Saudi arabian desert sand, Silicon 9(6), 917–922, https:// doi.org/10.1007/s12633-016-9531-8.
Plemyannikov, M. M. Zhdaniuk N. V. (2024). New vitreous materials and methods of their synthesis. Chemistry of vitreous materials. Kyiv, Igor Sikorsky Kyiv Polytechnic Institute.
Kamseu, Е. (2017). Substitution of sodium silicate with rice husk ash-NaOH solution in metakaolin based geopolymer cement concerning reduction in global warming, J. Clean. Prod. 142(4) 3050–3060, https://doi.org/10.1016/j. jclepro.2016.10.164.
Rao, B., Dai, H., Gao, L., Xie, H., Gao, G., Peng, K., Pan, Y. (2022). Surprisingly highly reactive silica that dissolves rapidly in dilute alkali (NaOH) solution even at ambient temperatures (25 °C). Journal of Cleaner Production, 341, 130779. https://doi.org/10.1016/j.jclepro.2022.130779.
Torres-Carrasco, M., Palomo, J.G., Puertas, F. (2014). Sodium silicate solutions from dissolution of glasswastes. Statistical analysis, Mater. Constr. 64(314), https://doi.org/10.3989/mc.2014.05213.
Prates, C. (2023). Use of iron ore tailing as raw material for two products: sodium silicate and geopolymers, J. Braz. Chem. Soc. 34(6), 809–818, https://doi. org/10.21577/0103-5053.20220149.
Lazaro, A.L., Rodríguez-Valadez, F.J., Lopez, J.J.M.H., Espejel-Ayala, F. (2020). SBA-15 synthesis from sodium silicate prepared with sand and sodium hydroxide, Mater. Res. Express, 7(4), https://doi.org/10.1088/2053-1591/ab83a5.
Ismail, A.A.М., Kannadasan, K., Pichaimani, P., Arumugam, H., Muthukaruppan, A. (2020). Synthesis and characterisation of sodium silicate from spent foundry sand: effective route for waste utilisation, J. Clean. Prod. 264, 121689, https://doi.org/10.1016/j.jclepro.2020.121689.
Pfeiffer, T., Enke, D., Roggendorf, H. (2021). Hydrothermal Dissolution of Technical Grade Vitreous Silica in NaOH Solutions to Liquid Water Glasses with Higher SiO2: Na2O Ratios. Chemie Ingenieur Technik. 93(3), 473–481. https://doi.org/10.1002/cite.202000107.
Febriana, E., Mayangsari, W., Yudanto, S.D., Sulistiyono, E., Handayani, M., Firdiyono, F., Maksum, A., Prasetyo, A.B., Soedarsono, J.W. (2024). Novel method for minimizing reactant in the synthesis of sodium silicate solution from mixed-phase quartz-amorphous SiO2. Case Studies in Chemical and Environmental Engineering, 9, 100656, https://doi.org/10.1016/j.cscee.2024.100656.
Chuiko, А. (2007). Structure and chemistry of silica surface. Kyiv, Scientific thought.
Torres-Carrasco, M., Palomo, J.G., Puertas, F. (2014). Sodium silicate solutions from dissolution of glasswastes. Statistical analysis, Mater. Constr. 64(314), https://doi.org/10.3989/mc.2014.05213.
Crundwell, F. K. (2017). On the mechanism of the dissolution of quartz and silica in aqueous solutions. ACS Omega 2, 1116–1127, https://doi.org/10.1021/acsomega.7b00019
Aphane, M.E., Doucet, F.J., Kruger, R.A., Petrik L., Van der Merwe, E.M. (2024). Preparation of sodium silicate solutions and silica nanoparticles from South African Coal Fly Ash, Waste and Biomass Valorization, 11, 4403–4417, https://doi.org/ 10.1007/s12649-019-00726-6.
Pipathworapoom, W., Hsu, H.P., Manibalan, K., Lan, C.W. (2022). Rapid synthesis of sodium silicate through adiabatic reaction with low emission starting from exhausted KL-Si, J. Clean. Prod., 376, 134298, https://doi.org/10.1016/j. jclepro.2022.134298
Owoeye, S.S., Jegede, F.I., Borisade, S.G. (2020). Preparation and characterization of nanosized silica xerogel particles using sodium silicate solution extracted from waste container glasses, Mater. Chem. Phys., 248, 122915, https://doi.org/10.1016/j.matchemphys.2020.122915.
Mayangsari, W., Prasetyo, A.B., Febriana, E., Maksum, A., Subagja, R., Firdiyono, F., Wahyuadi, J. (2023). The effect of mixing methods before the decomposition process of ferronickel slag, Acta Metall. Slovaca. 29(4), 187–191, https://doi.org/10.36547/ams.29.4.1925
Figueiredo, R.A.M., Brandao, P.R.G., Soutsos, M., Henriques, A.B., Fourie, A., Mazzinghy, D. B. (2021). Producing sodium silicate powder from iron ore tailings for use as an activator in one-part geopolymer binders, Mater. Lett. 288. 129333, https://doi.org/10.1016/j.matlet.2021.129333.
Alam, Q., Hendrix, Y., Thijs, L., Lazaro, A., Scholbach, K., Brouwers, H.J.H. (2019). Novel low temperature synthesis of sodium silicate and ordered mesoporous silica from incineration bottom ash, J. Clean. Prod. 211: 874–883, https://doi.org/10.1016/j.jclepro.2018.11.173.
Brindley, G., Brown, G. (1980). Crystal structures of clay minerals and their X-ray indentification. London: Miner.
Plemiannikov, M.M., Zhdaniuk, N.V. (2021). Ferrosilicate glass ceramics based on wastes from ore concentration. Issues of Chemistry and Chemical Technology. 2, 95–103. doi:10.32434/0321-4095-2021-135-2-95-103
Zhdaniuk, N. V. (2023). Study of the possibility of recycling waste from metallurgical productions using glass technology. Publishing House «Baltija Publishing».
Lafuente, B., Downs, R. T., Yang, H., Stone, N., Armbruster, T., Danisi, R. M. (2015). The power of databases: the RRUFF project. Highlights in mineralogical crystallography, 1, 25.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

