VISIBLE LIGHT ACTIVE FLUORITE-TYPE NANOCOMPOSITES FORMED IN THE CeO2-La2O3-Dy2O3 SYSTEM
DOI:
https://doi.org/10.15421/jchemtech.v32i4.311116Keywords:
nanocomposite, CeO2-La2O3-Dy2O3, fluorite-type structure, visible light photocatalytic activity, Malachite Green, dye destructionAbstract
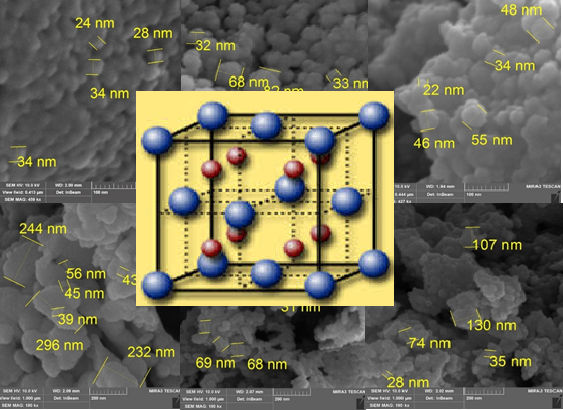
Fluorite-type nanocomposite materials with a content of 95–60 mol.% CeO2, 10–20 mol.% La2O3, and 5–20 mol.% Dy2O3 were obtained by the chemical co-precipitation method using REЕ (rare earth elements) nitrate solutions. The primary particle size (coherent scattering region) increased from 11.3 to 26.5 nm when the calcination temperature increased from 600 to 800 °C. The highest photocatalytic activity in the destruction of Malachite green dye under the influence of visible light was shown by the sample with the composition of CeO2 (80 mol.%) - La2O3 (10 mol.%) - Dy2O3 (10 mol.%), thermally treated at 600 °C for 5 hours. The results of the experimental study showed that at the initial concentration of the Malachite green (MG) solution of 20 mg/dm3 under the influence of visible light for 1 hour, its residual concentration was only 0.45 mg/dm3, and the degree of decolorization of the solution reached 98 %. It is assumed that nanocomposites based on a CeO2-La2O3-Dy2O3 solid solution with a fluorite-type structure can be used to create effective photocatalysts designed for the destruction of cationic dyes in an aqueous medium.
References
Zeng, S., Shui, A., Yu, H., He, C. (2024). Sonochemical synthesis of CeO2 nanoparticles with high photocatalytic and antibacterial activities under visible light. Applied Ceramic Technology, 21, 3141–3151. https://doi.org/10.1111/ijac.14775
Xu, Y., Zhou, Y., Li, Y., Liu, Y., Ding, Z. (2024). Advances in cerium dioxide nanomaterials: Synthesis, strategies, property modulation, and multifunctional applications. Journal of Environmental Chemical Engineering, 12(5), 113719. https://doi.org/10.1016/j.jece.2024.113719
Momin, Naeemakhtar., Manjanna, J., D′Souza, L., Aruna, S.T., Sentil Kumar, S. (2022). Synthesis, structure and ionic conductivity of nanocrystalline Ce1−xLaxO2−δ as an electrolyte for intermediate temperature solid oxide fuel cells. Journal of Alloys and Compounds, 896, 163012. https://doi.org/10.1016/j.jallcom.2021.163012
Li, C., Zheng, Y., Li, M., Fang, B., Lin, J., Ni, J., Wang, X., Lin, B., Jiang, L. (2022). Enhancement of ammonia synthesis activity on La2O3-supported Ru catalyst by addition of ceria. International Journal of Hydrogen Energy, 47(55), 23240–23248. https://doi.org/10.1016/j.ijhydene.2022.05.133
Zhang, Y., Yan, Z., Xiao, M., Zhang, C., Ruan, L., Zhang, Y., Zhong, Y., Yan, Y., Yu, Y., He, H. (2025). Catalytic performance of Pd catalyst supported on CeO2 or ZrO2 modified beta zeolite for methane oxidation. Journal of Environmental Sciences, 152, 248–261. https://doi.org/10.1016/j.jes.2024.05.005
Chen, J. P., Patil, S., Seal, S., McGinnis, J. F. (2006). Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nature Nanotechnology, 1, 142–150. https://doi.org/10.1038/nnano.2006.91
van Deelen, T.W., Hernández Mejía, C., de Jong, K.P. (2019). Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nature Catalysis, 2(11), 955–970. https://doi.org/10.1038/s41929-019-0364-x
Joshi, A., Chand, P., Saini, S. (2023). Improved electrochemical performance of rare earth doped Bi1-xMxPO4 (x=0, 0.15; M=La, Ce, Sm) Nanostructures as electrode material for energy storage applications. Journal of Alloys and Compounds, 935(2), 168063. https://doi.org/10.1016/j.jallcom.2022.1680639.
9.Stamatelos, I., Scheepers, F., Pasel, J., Dinh, C.-T., Stolten, D. (2024). Ternary Zn-Ce-Ag catalysts for selective and stable electrochemical CO2 reduction at large-scale. Applied Catalysis B: Environment and Energy, 353, 124062. https://doi.org/10.1016/j.apcatb.2024.124062
Wu, Q., Fu, H., Dang, C., Yang, G., Cao, Y., Wang, H., Wang, H.-F., Yu, H. (2024). In-modified Ni-CeO2 for reverse water-gas shift: Cooperation between oxygen vacancies and intermetallic compounds. Chemical Engineering Science, 299(5), 120547. https://doi.org/10.1016/j.ces.2024.120547
Shukla, M.K., Balyan, Y., Kumar, A., Bhaskar, T., Dhar, A. (2022). Catalytic oxidation of soot by CeO2-ZrO2 catalysts: Role of Zr. Materials Chemistry and Physics, 286(1), 126161. https://doi.org/10.1016/j.matchemphys.2022.126161
Wang, C., Huang, W., Wang, Y., Cheng, Y.L., Zou, B.L., Fan, X.Z., Yang G.L., Cao, X.Q. (2012). Synthesis of monodispersed La2Ce2O7 nanocrystals via hydrothermal method: A study of crystal growth and sintering behavior. International Journal of Refractory Metals and Hard Materials, 31, 242–246. 10.1016/j.ijrmhm.2011.12.002
Liying, H.E., Yumin, S.U., Lanhong, Jiang., Shikao, S.H.I. (2015). Recent advances of cerium oxide nanoparticles in synthesis, luminescence and biomedical studies: a review. Journal of Rare Earths, 33(8), 791–799. https://doi.org/10.1016/S1002-0721(14)60486-5
Kandasamy, P., Kandala, B., Wook Lee, M., Sivakumar, G. (2024). Phase stability and initial phase high-temperature corrosion behavior of non-stoichiometric lanthanum cerium oxide thermal barrier coatings. Ceramics International, 50(9), 14458–14468. https://doi.org/10.1016/j.ceramint.2024.01.358
Chen, K., Wan, J., Wang, T., Sun, Qi., Zhou, R. (2023). Construction of bimetallic Pt–Pd/CeO2–ZrO2–La2O3 catalysts with different Pt/Pd ratios and its structure–activity correlations for three-way catalytic performance. Journal of Rare Earths, 41(6), 896–904. https//doi.org/10.1016/j.jre.2022.10.014
Seminko, V.V., Maksimchuk, P.O., Sedyh, O.O., Aslanov, A.V., Malyukin, Yu. V. (2020). Improving •OH scavenging properties of nanoceria by doping and pre-irradiation. Functional Materials, 27(1), 6–11.
doi: 10.15047/fm27.01/06
Zarur, A.J., Ying, J.Y. (2000). Reverse microemulsion synthesis of nanostructured complex oxides for catalytic combustion. Nature, 403, 65–67. https://doi.org/10.1038/47450
Schicks, J., Neumann, D., Specht, U., Veser, G. (2003). Nanoengineered catalysts for high-temperature methane partial oxidation, Catalysis Today, 81(2), 287–296. https://doi.org/10.1016/S0920-5861(03)00116-0
Coduri, M., Checchia, S., Longhi, M., Ceresoli, D., Scavini, M. (2018). Rare Earth Doped Ceria: The Complex Connection Between Structure and Properties. Frontiers Chemistry, 6, 526. doi:10.3389/fchem.2018.00526
Zheng, Y., Zhou, M., Ge, L., Li, Sh., Chen, H., Guo, L. (2011). Effect of Dy on the properties of Sm-doped ceria electrolyte for IT-SOFCs. Journal of Alloys and Compounds, 509(4), 1244–1248. doi:10.1016/j.jallcom.2010.09.203
Jagjeet, K., Deepika, Ch., Vikas, D., Ravi, Sh., Yogita, P., Suryanarayana, N. S. (2016). Photoluminescence Characteristics of Dysprosium Doped CeO2 Phosphor for White Light Emission. J. Display Technol, 12, 506–512. https://opg.optica.org/jdt/abstract.cfm?URI=jdt-12-5-506
Trovarelli, A., and Llorca, J. (2017). Ceria catalyst at the nanoscale: How do crystal shapes shape catalysis? ACS Catal, 7, 4716–4735. doi: 10.1021/acscatal.7b01246
Salehi, Z., Zinatloo-Ajabshir, S., Salavati-Niasari, M. (2017). Dysprosium cerate nanostructures: facile synthesis, characterization, optical and photocatalytic properties. Journal of Rare Earths, 35(8), 805–812. doi: 10.1016/S1002-0721(17)60980-3
Zinatloo-Ajabshir, S., Salehi, Z., Salavati-Niasari, M. (2018). Green synthesis and characterization of Dy2Ce2O7 nanostructures using Ananas comosus with high visible-light photocatalytic activity of organic contaminants. Journal of Alloys and Compounds, 763, 314–321. https://doi.org/10.1016/j.jallcom.2018.05.311
Kornienko, O.A., Andrievskaya, O.R., Barshchevskaya, H. K. (2020). [Phase relations in the system ternary based on ceria, zirconia and ytterbia at 1500 °С]. Journal of Chemistry and Technologies, 28(2), 142–152. https://doi.org/10.15421/082015 (In Ukrainian)
Korniienko, O.A., Yushkevich, S.V., Bykov, O.I., Samelyuk, A.V., Bataiev, Yu.M., Zamula M.V. (2022). Phase equilibrium in binary La2O3-Dy2O3 and ternary CeO2-La2O3-Dy2O3 systems. Journal of the European Ceramic Society, 42(13), 5820–5830 https://doi.org/10.1016/j.jeurceramsoc.2022.06.045
Yushkevych, S.V., Korniienko, O.A., Bykov, O.I., Subota, I.S. (2023). Isothermal section for the ternary CeO2–Lа2O3–Dy2O3 system at 1100 °С. Journal of Chemistry and Technologies, 31(2), 215–222. https://doi.org/10.15421/jchemtech.v31i2.275434
Torun, H.Ö., Kırkgeçit, R., Dokan, F.K., Öztürk, E. (2021). Preparation of La-Dy-CeO2 ternary compound: Examination of photocatalytic and photoluminescence properties. Journal of Photochemistry and Photobiology A: Chemistry, 418, 113338. https://doi.org/10.1016/j.jphotochem.2021.113338
Lavrynenko, O.M., Pavlenko, O.Yu., Zahornyi, M.N., Korichev, S.F. Morphology, phase and chemical composition of the nanostructures formed in the systems containing lanthanum, cerium, and silver. (2021). Chemistry, Physics and Technology of Surface, 12, 4, 382–392. doi: 10.15407/hftp12.04.382
Zahornyi, M.M., Lavrynenko, O.M., Kolomys, O.F., Strelchuk, V.V., Tyschenko, N.I., Korniienko, O.A., Ievtushenko, A.I. (2023). Modern Photoactive Nanocomposites Based on TiO2 and CeO2. Journal of Nano-And Electronic Physics, 15(4), 04001. https://doi.org/10.21272/jnep.15(4).04001
Lavrynenko, O.M., Zahornyi, M.M., Vember, V.V., Pavlenko, O.Yu., Lobunets, T.F., Kolomys, O.F., Povnitsa, O.Yu., Artiukh, L.O., Naumenko, K.S., Zahorodnia, S.D., Garmasheva, І.L. (2022). Nanocomposites based on cerium, lanthanum, and titanium oxides doped with silver for biomedical application. Condens. Matter (MDPI), 7(3), 45. https://doi.org/10.3390/condmat7030045
"Atomic and Ionic Radius". Chemistry LibreTexts. (2013). https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Atomic_and_Ionic_Radius
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

