SYNTHESIS AND ELECTRICAL CONDUCTIVITY OF FLUORIDE-CONDUCTING PHASES SrSnF4 AND PbxSr1-xSnF4
DOI:
https://doi.org/10.15421/jchemtech.v33i1.311813Keywords:
fluoride-conducting phases, tin, strontium, lead fluorides, synthesis, electrical conductivityAbstract
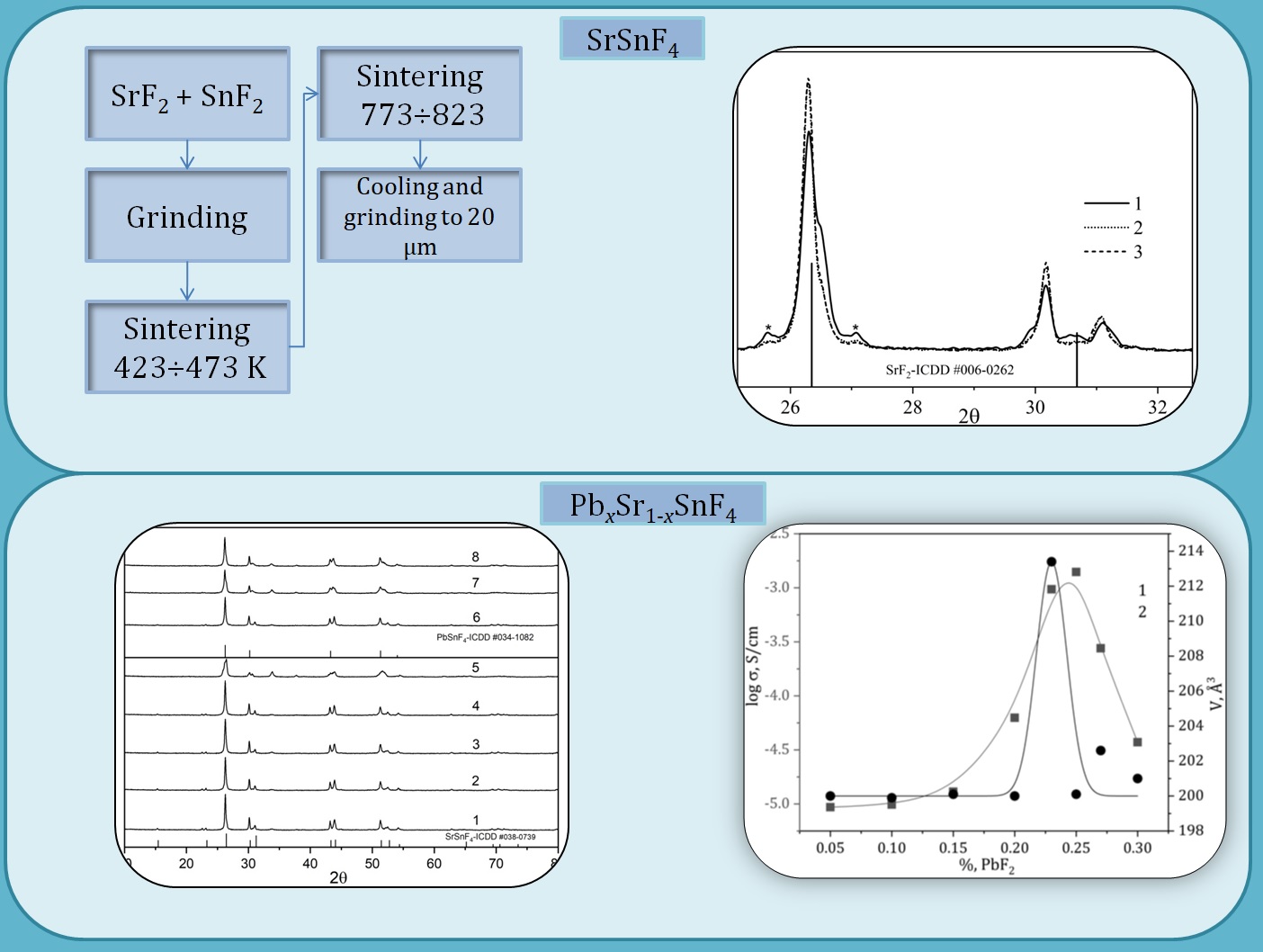
In the article it is proposed to synthesize free of impurity phases complex fluoride SrSnF4 by sintering lead and strontium fluorides in an equivalent ratio at temperatures higher than temperature of its phase transition from cubic modification into tetragonal (623 K). The duration of synthesis to obtain SrSnF4 free from impurity phases at 773 K is 1 hour, and at 823 K – half an hour. Synthesis at temperatures lower than temperature of phase transition is associated with the formation of impurity phases different in composition and structure from SrSnF4. The conductivity of such samples at 293 K is almost an order of magnitude higher than the conductivity of SrSnF4, which is free from impurity phases (1.18∙10-5 S/cm, Ea = 0.28 eV). The synthesis of fluoride-conducting phases PbxSr1-xSnF4 is proposed to be carried out by the method of sintering the initial components in a given ratio in two stages. First, the initial mixture is sintered in the temperature range of 423÷473 K for an hour. Then the temperature is raised to 773÷823 K and the reaction mixture is sintered for another hour. The crystal lattice of PbxSr1-xSnF4 samples of solid solutions of the isovalent substitution synthesized in this way, with the content of the substituent of 0 ˂ x £ 0.25, corresponds to tetragonal lattice (P4/nmm) and is similar to SrSnF4. At a higher content of the substituent (0.25 ˂ x £ 0.30), the symmetry of the crystal lattice does not change, but corresponds to another structural type (β-PbSnF4). The highest conductivity (1.12∙10-3 S/cm at 293 K) and the lowest activation energy (0.063 eV) has the Pb0.25Sr0.75SnF4 phase, which corresponds to the region where the structural type of the crystal lattice is rearranged with increasing of content of the substituent.
References
Gschwind, F., Rodriguez-Garcia, G., Sandbeck, D. J. S., Gross, A., Wiel, M., Fichtner, M., Hörmann, N. (2016). Fluoride ion batteries: theoretical performance, safety, toxicity, and a combinatorial screening of new electrodes. J. Fluor. Chem., 182, 76–90. https://doi.org/10.1016/j.jfluchem.2015.12.002
Xiao, A., Galatolo, G., Pasta, M. (2021). The case for fluoride-ion batteries. Joule, 5, 2823–2844. https://doi.org/10.1016/j.joule.2021.09.016
Nowroozi, M. A., Mohammad, I., Molaiyan, P., Wissel, K., Reddy, A. M., Clemens, O. (2021). Fluoride ion batteries – past, present, and future. J. Mater. Chem., 9, 5980–6012. https://doi.org/10.1039/D0TA11656D
Patro, L. N., Hariharan, K. (2013). Fast fluoride ion conducting materials in solid state ionics: An overview. Solid State Ionics, 239, 41–49. https://doi.org/10.1016/j.ssi.2013.03.009
Tingting, L., Na, P., Xikun, Z., Runtian, Z., Maoting, X., Jundong, Z., Haoxiang, Y., Liyuan, Z., Jie, S. (2021). Insight into anion storage batteries: Materials, properties and challenges. Energy Storage Materials, 42, 42–67. https://doi.org/10.1016/j.ensm.2021.07.011
Mohammad, I., Chable, J., Witter, R., Fichtner, M., Reddy, M. A. (2018). Synthesis of Fast Fluoride-Ion-Conductive Fluorite-Type Ba1−xSbxF2+x (0.1 ≤ x ≤ 0.4): A Potential Solid Electrolyte for Fluoride-Ion Batteries. ACS Appl. Mater. Interfaces, 10(20), 17249–17256. https://doi.org/10.1021/acsami.8b04108
Zhang, L., Reddy, M. A., Fichtner, M. (2015). Development of tysonite-type fluoride conducting thin film electrolytes for fluoride ion batteries. Solid State Ionics, 272, 39–44. https://doi.org/10.1016/j.ssi.2014.12.010
Mori, K., Morita, Y., Saito, T., Kamiyama, T., Otomo, T., Abe, T., Fukunaga, T. (2020). Structural and Electrochemical Properties of Tysonite Ce0.95A0.05F2.95 (A = Mg, Ca, Sr, and Ba): Fast-Fluoride-Ion-Conducting Solid Electrolytes. J. Phys. Chem., 124(34), 18452–18461. https://doi.org/10.1021/acs.jpcc.0c05217
Qianlong, J., Melnikova, N. A., Glumov, O. V., Trefilov, I. O., Eliseeva, S. N., Murin, I. V. (2023). Mechanochemical synthesis, microstructure and electrochemical properties of solid electrolytes with stabilized fluorite-type structure in the PbF2-SrF2-KF system for solid-state fluoride-ion batteries. Ceramics International, 49(11), 16901–16908. https://doi.org/10.1016/j.ceramint.2023.02.051
Ahmad, M. M., Yamane, Y., Yamada, K. (2013). The ionic conductivity and dielectric properties of Ba1-xSnxF2 solid solutions prepared by mechanochemical milling. Materials Science and Engineering B, 178, 965–970. https://doi.org/10.1016/j.mseb.2013.05.011
Molaiyan, P., Witter, R. (2019). Crystal phase and surface defect driven synthesis of Pb1−xSnxF2 solid solution electrolyte for fluoride ion batteries. Journal of Electroanalytical Chemistry, 845, 154–159. https://doi.org/10.1016/j.jelechem.2019.04.063
Pogorenko, Yu. V, Nahornyi, A. A., Pshenichnyi, R. M., Omelchuk, A. O. (2019). Synthesis and electrical conductivity of solid solutions of the RbF–PbF2–SnF2 system. Ukrainian Chemistry Journal, 85(7), 60-68. https://doi.org/10.33609/0041-6045.85.5.2019.60-68
Callanan, J. E., Wesrum, R. S., Wesrum, E. F., Weirs, R. D. (1989). The Thermodynamics of the Divalent Metal Fluorides. Ill. Heat Capacity of the Fast Ion Conductor SrSnF4, from 6 to 344 K. Journal of Solid State Chemistry, 81, 51–57. https://doi.org/10.1016/0022-4596(89)90200-4
Katapalli, R. A., Yenduri, B. R., Ramesh, K. K., Laxmi, N. P. (2023). Mechanochemical Synthesis and Fluoride Ion Conductivity Studies in SrSnF4 Polymorphs. J. Phys. Chem. C., 127(16), 7816–7822. https://doi.org/10.1021/acs.jpcc.3c00056
Hull, S. (2004). Superionics: crystal structures and conduction. Rep. Prog. Phys., 67, 1233–1314. https://doi.org/10.1088/0034-4885/67/7/R05
Lei, L., Li, Y., Dingsheng, S., Kaili, L., Changfei, Z., Zhigao, L., Xianyou, W. (2020). Nd3+ doped BaSnF4 solid electrolyte for advanced room-temperature solid-state fluoride ion batteries. Ceramics International, 46(12), 20521–20528. https://doi.org/10.1016/j.ceramint.2020.05.161
Nahornyi, A. A., Voloshanovska, Yu. V., Omelchuk, A. O. (2022). Electrical conductivity of solid fluoride phases composition BaxPb0.86‐xSn1.14F4. Ukrainian Chemistry Journal, 88(11), 39–54. https://doi.org/10.33609/2708-129X.88.11.2022.39-54
Lei, L., Li, Y., Min, L., Xiaolong, L., Dingsheng, S., Kaili, L., Xianyou, W., Zhigao, L. (2020). SnF2-based fluoride ion electrolytes MSnF4 (M = Ba, Pb) for the application of room temperature solid-state fluoride ion batteries. Journal of Alloys and Compounds, 819, 152983. https://doi.org/10.1016/j.jallcom.2019.152983
Mohammad, I., Witter, R., Fichtner, M., Reddy, M. A. (2019). Introducing Interlayer Electrolytes: Toward Room-Temperature High-Potential Solid-State Rechargeable Fluoride Ion Batteries. ACS Appl. Energy Mater., 2(2), 1553–1562. https://doi.org/10.1021/acsaem.8b02166
Mercadier, B., Coles, S. W., Duttine, M., Legein, C., Body, M., Borkiewicz, O. J., Lebedev, O., Morgan, B. J., Masquelier, C., Dambournet, D. (2023). Dynamic Lone Pairs and Fluoride-Ion Disorder in Cubic-BaSnF4. J. Am. Chem. Soc., 145(43), 23739–23754. https://doi.org/10.1021/jacs.3c08232
Qiaoju, N.,Yaowei, H., Lin, Ch., Yudong, F., Gang, W., Ming, Z., Zhongrong, S. (2024). Effect of moisture on the phase transition of β-PbSnF4 at ambient temperature as the fast fluoride ion conductor. Solid State Ionics., 405, 116454. https://doi.org/10.1016/j.ssi.2024.116454
Shannon, R. D. (1976). Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst., B25, 925–945. https://doi.org/10.1107/S0567739476001551
Data from All Phase Diagrams http://www.crct.polymtl.ca/fact/documentation/FS_All_PDs.htm
Crystal Impact. Software for Scientists. Match, Phase Analysis using Powder Diffraction http://www.crystalimpact.de/match/
Petricek, V., Palatinus, L., Plasil, J., Dusek, M. (2023). Jana2020 - a new version of the crystallographic computing system Jana. Z. Kristallogr., 238(7-8), 271–282. https://doi.org/10.1515/zkri-2023-0005
Donaldson, J. D., Senior, B. J. (1967). Fluorostannates(II): the non-transition-metal(II) derivatives of the complex tin(II) fluoride ions. Chem. Soc. A, 1821–1825.
Irvine, J. T. S., Sinclair, D. C., West, A. R. (1990). Electroceramics: Characterization by impedance spectroscopy. Adv. Mater., 2, 132−138. https://doi.org/10.1002/adma.19900020304
Almond, D. P., West, A. R. (1983). Mobile ion concentrations in solid electrolytes from an analysis of a.c. conductivity. Solid State Ionics, 9−10, 277−282. https://doi.org/10.1016/0167-2738(83)90247-3
Pohorenko, Yu. V., Pshenychnyi, R. M., Omelchuk, A. O., Trachevskii, V. V. (2019). Conductivity of solid solutions of heterovalent substitution Pb1-xLnxSnF4+x (Ln=Y, La, Ce, Nd, Sm, Gd) with β-PbSnF4 structure. Solid State Ionics, 338, 80–86. https://doi.org/10.1016/j.ssi.2019.05.001
Pogorenko, Yu. V., Pshenychnyi, R. M., Omelchuk, A. O., Lutsyk, V. I. (2017). Transport Properties of Aliovalent Substitution Solid Solutions of the System (1-x)PbF2-xYF3-SnF2. IOP Conferense Series: Materials Science and Engineering, 175, 1–6. doi:10.1088/1757-899X/175/1/012039 =
Kazuhiro Mori, Shuki Torii, Kenji Iwase, Takeshi Abe, Toshiharu Fukunaga (2023). Effects of Mixed Phases on Electrical Conductivities for (CeF3)1–m(CaF2)m Fast-Fluoride-Ion-Conducting Solid Electrolytes. J. Phys. Chem., 127, 59−68. https://doi.org/10.1021/acs.jpcc.2c06732
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

