PHYSICO-CHEMICAL STUDY, MOLECULAR MODELING AND ANTIBACTERIAL ACTIVITY OF α- AND β-ANOMERS OF XYLOSE ESTERS
DOI:
https://doi.org/10.15421/jchemtech.v33i1.312010Keywords:
Xylose ester; anomers; surface properties; modeling; antibacterial activityAbstract
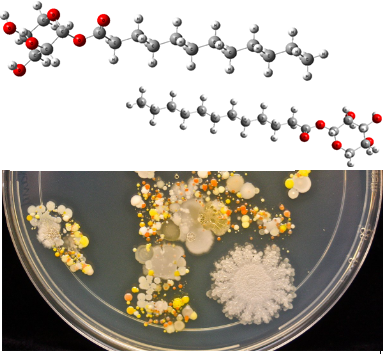
Non-ionic surfactants obtained from renewable resources represent a new challenge in biotechnology and have a range of applications in many industries. In this context, the α- and β-anomers of synthesized D-xylose-based bio-surfactants with various chain length were easily separated and characterized through analytical methods. Their surface and emulsifying properties were evaluated. To study the reactivity of the two anomers of synthetized biosurfactants, the spatial conformations of the prepared acyl-xylopyranose were obtained by molecular modeling with Gaussian 9 software using the density functional theory method. Compounds α are the softest so more reactive than the β ones. The antibacterial activity of sugar fatty acid ester anomers was also studied. The results obtained indicate the importance of anomeric form and chain length for the stability of the synthesized compounds.
References
Perinelli, D.-R., Lucarini, S., Fagioli, L., Campana, R., Vllasaliu, D., Duranti, A., Casettari, L. (2018). Lactose oleate as new biocompatible surfactant for pharmaceutical application. Eur J Pharm Biopharm. 124, 55–62. doi.10.1016/j.ejpb.2017.12.008.
Marathe, S.-J., Dedhia, N., Singhal, R.-S. (2022). Esterification of sugars and polyphenols with fatty acids: techniques, bioactivities, and applications. Curr. Opin. Food Sci., 43, 163–173. 10.1016/j.cofs.2021.12.008.
Tiboni, M., Elmowafy, E., El-Derany, M.-O., Benedetti, S., Campana, R., Verboni, M., Potenza, L., Palma, F., Citterio, B., Sisti, M.; Duranti, A., Lucarini, S., Soliman, M.-E., Casettari, L. A. (2022). Combination of sugar esters and chitosan to promote in vivo wound care. International Journal of Pharmaceutics, 616, 121508. 10.1016/j.ijpharm.2022.121508.
Chaiwut, P., Jirarat, A., Tiensri, N., Sangthong, S., Pintathong, P. (2022). Green Synthesis Optimization of Glucose Palm Oleate and Its Potential Use as Natural Surfactant in Cosmetic Emulsion. Cosmetics, 9(4), 76. 10.3390/cosmetics9040076.
Kennedy, J.-F., Kumar, H., Panesar, P.-S., Marwaha, S.-S., Goyal, R., Parmar, A., Kaur, S. (2006). Enzyme-catalyzed regioselective synthesis of sugar esters and related compounds. Journal of Chemical Technology & Biotechnology, 81, 866–876. doi: 10.1002/jctb.1473.
Gumel, A.-M., Annuar, M.S.M., Heidelberg, T., Chisti, Y. (2011). Lipase mediated synthesis of sugar fatty acid esters. Process Biochemistry, 46, 2079–2090. doi: 10.1016/j.procbio.2011.07.021
Vuillemin, M.-E., Husson, E., Laclef, S., Jamali, A., Lambertyn, V., Pilard, S., Cailleu, D., Sarazin, C. (2022). Improving the environmental compatibility of enzymatic synthesis of sugar-based surfactants using green reaction media. Process Biochemistry, 117, 30–41. doi: 10.1016/j.procbio.2022.03.015.
Klibanov, A. (2001). Improving enzymes by using them in organic solvents. Nature, 409, 241–246. doi: 10.1038/35051719.
Bidjou-Haiour, C., Klai, N. (2013). Lipase Catalyzed Synthesis of Fatty Acid Xylose Esters and their Surfactant Properties. Asian Journal of Chemistry, 25, 4347–4350. doi: 10.14233/ajchem.2013.13973.
Sharma, R.-K., Ggarwal, N., Arya, A., Olsen, C.-E., Parmar, V.-S., Prasad, A.-K. (2009). Lipase-catalyzed regio-and stereoselective deacylation: Separation of anomers of peracylated a,ß-D-ribofuranosides. Indian Journal of chemistry, 48, 1727–1731.
Jabbari, H., Khosravi, S., Azimi, S. (2022). Synthesis and Evaluation of New Derivatives of Busulfan as an Anti-carcinogenic Drug against k562 Cancer Cells Using the AO / PI Method. The Open Medicinal Chemistry Journal, 16, 1–9, doi: 10.2174/18741045-v16-e2209140.
Frisch, M.-J.; Trucks, G.-W.; Schlegel, H.-B.;
Scuseria, G.-E.; Robb, M.-A.; Cheeseman, J.-R.; Scalmani, G.; Barone, V.; Petersson, G.-A.; Nakatsuji, H. Gaussian 09 (2013). Gaussian, Inc. (Wallingford CT).
Zhao, L., Zhang, H., Hao, T., Li., S. (2015). In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chemistry, 187, 370–377. https://doi.org/10.1016/j.foodchem.2015.04.108
Klai, N., Bidjou-Haiour, C., Bouquillon, S. (2015). D-Xylose-based surfactants: Synthesis, characterization and molecular modeling studies. Comptes Rendus Chimie, 18, 599–606. doi: 10.1016/j.crci.2014.11.007.
Razafindralambo, H., Blecker, C., Mezdour, S., Deroanne, C., Crowet, J.M., Brasseur, R., Paquot, M. (2009). Impacts of the Carbonyl Group Location of Ester Bond on Interfacial Properties of Sugar-Based Surfactants: Experimental and Computational Evidences. The Journal of Physical Chemistry B, 113, 8872–8877. doi: 10.1021/jp903187f.
Larsson, J., Sanchez-Fernandez, A., Mahmoudi, N., Barnsley, L., Wahlgren, M., Nylander, T., Ulvenlund, S. (2019). The effect of the anomeric configuration on the micellization of hexadecylmaltoside surfactants. Langmuir, 35, 13904–13914. doi: 10.1021/acs.langmuir.9b01960.
Zhang, X., Wei, W., Cao, X., Feng, F. (2014). Characterization of enzymatically prepared sugar medium-chain fatty acid monoesters. Journal of the Science of Food and Agriculture, 95, 1631–1637. doi: 10.1002/jsfa.6863.
Jiang, Y., Xu, Y., Li, F., Li, D., Huang, Q. (2019). Pectin Extracted from Persimmon Peel: A Physicochemical Characterization and Emulsifying Properties Evaluation. Food Hydrocolloids, 101, 105561. doi: 10.1016/j.foodhyd.2019.105561.
Zhang, X.; Song, F.; Taxipalati, M.; Wei, W., Feng, F. (2014). Comparative Study of Surface-active Properties and Antimicrobial Activities of Disaccharide Monoesters. PLoS one, 9, e114845. doi: 10.1371/journal.pone.0114845
Frisch, M.-J., Trucks, G.-W.; Schlegel, H.-B., et al. (2010). Gaussian 09, Revision B.01 and Revision D.01. Gaussian, Inc., Wallingford CT.
Stephens, P.J., Devlin, F.J., Chabalowski, C.F., Frisch, M.J. (1994). Ab Initio Calculation of Vibrational Absorption and Circula Dichroism Spectra Using Density Functional Force Fields. Journal of Physical Chemistry B, 98, 11623–11627. doi: 10.1021/j100096a001.
Hay, P.J. (1977). Gaussian basis sets for molecular calculations. The representation of 3d orbitals in transition-metal atoms. Journal of Chemical Physics, 66, 4377–4384. doi: 10.1063/1.433731.
Bauer, A.W., Kirby, W.M.M., Sherris, J.C., Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical, 45, 493–496. DOI: 10.1093/ajcp/45.4_ts.493
(2020). Performance standards for antimicrobial susceptibility testing. M 100, 30th ed. 126–128. https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf
Ppeum Ki, L.; Hyung Kwoun, K. (2016). Antibacterial effect of fructose laurate synthesized by Candida antarctica B lipase-mediated transesterification. Journal of Microbiology and Biotechnology, 26(9). 1579–1585, doi:https://doi.org/10.4014/jmb.1601.01045
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

