HIRSHFELD SURFACE ANALYSIS OF A MONONUCLEAR PYRAZOLE-CONTAINING COPPER(II) COMPLEX OBTAINED BY OXIDATIVE DISSOLUTION METHOD
DOI:
https://doi.org/10.15421/jchemtech.v33i2.312363Keywords:
pyrazole ligands, copper complexes, crystal structure, oxidative dissolution, Hirshfeld surface analysisAbstract
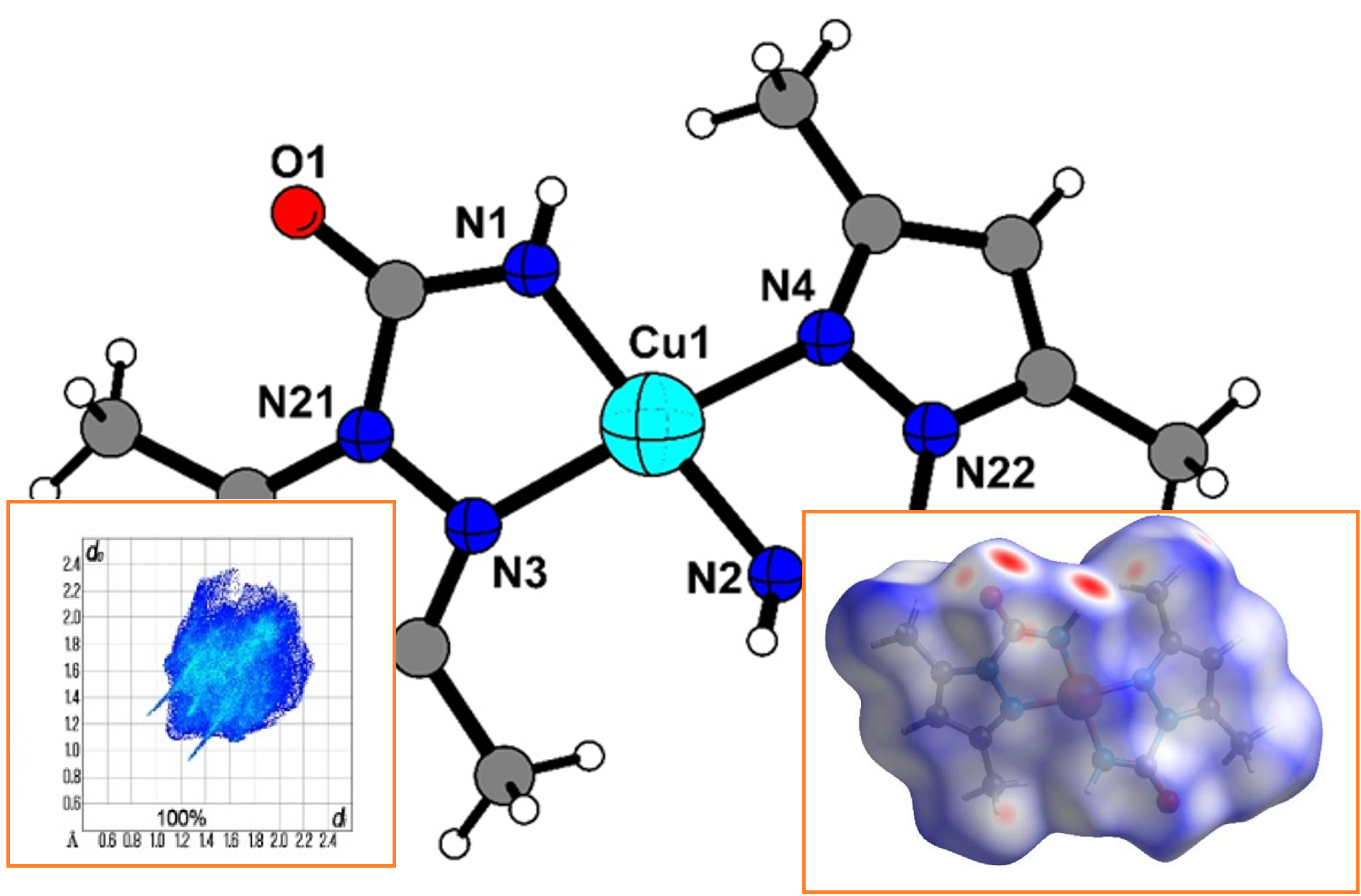
In this work, a method of oxidative dissolution was used to obtain mononuclear copper(II) complex Cu(С6H8N3O)2 with 1-carboxamide-3,5-dimethylpyrazole. The variety of techniques were used to identify and characterize the structure of the complex and the ligand, such as IR and NMR spectroscopy, microanalyses, single-crystal X-ray diffraction. Hirshfeld surface analysis was performed to visualize the close intermolecular atomic contacts in the crystal structure of the title compound. It was found that Cu(С6H8N3O)2 was formed as a result of a multistage process of oxidative dissolution of metallic copper with the participation of air oxygen and ammonium ions. This process leads to the appearance of a sufficient amount of Cu2+ ions in the solution, which partially ensures the production of the final compound, as well as the dissolution of zero-valent copper through the formation of intermediate Cu1+ compounds, which are oxidized by air oxygen to form divalent copper compounds. It has also been found that ammonium cyanate is formed in parallel through exchange reactions, which undergo dissociation in acetonitrile solutions, and the resulting HNCO undergoes hydrolysis. The hydrolysis products react with the starting ligand 3,5-dimethyl-1H-pyrazole to form 1-carboxamide-3,5-dimethylpyrazole. This acid then reacts with Cu2+ ions to form the final mononuclear complex. Single‐crystal X‐ray diffraction analysis reveals that the title compound crystallizes in the triclinic crystal system, space group P (Z = 2). For the title compound, the most significant contributions to the overall crystal packing are from H···H (47 %), H···O/O···H (19.5 %), H···C/C···H (12.1 %) and H···N/N···H (11.5 %) contacts. According to the Hirshfeld surface analysis, hydrogen bonds (H···H and H···O/O···H) and other close contacts involving hydrogen atoms make the main contribution to intermolecular interactions in the title compound. Two intermolecular NH···O contacts with a length of 2.192 Å are the shortest.
References
Costa, R. F., Turones, L. C., Cavalcante, K. V. N., Rosa Júnior, I. A., Xavier, C. H., Rosseto, L. P., Fajemiroye, J. O. (2021). Heterocyclic compounds: pharmacology of pyrazole analogs from rational structural considerations. Frontiers in Pharmacology, 12, 666725. https://doi:10.3389/fphar.2021.666725
Kamel, M. (2015). Convenient Synthesis, Characterization, Cytotoxicity and Toxicity of Pyrazole Derivatives. ACSi 62(1), 136–151. https://doi:10.17344/acsi.2014.828
Clemett, D., and Goa, K. L. (2000). Celecoxib. Drugs, 59, 957–980. https://doi:10.2165/00003495-200059040-00017
Samat, A., Tomlinson, B., Taheri, S., and Thomas, G. (2008). Rimonabant for the Treatment of Obesity. Recent Pat. Cardiovasc. Drug Discov., 3(3), 187–193. https://doi:10.2174/157489008786264014
Straube, S. (2012). Anti-inflammatory and Antipyretic Analgesics and Drugs Used in Gout. Side Effects Drugs Annu., 181–193. https://doi:10.1016/b978-0-444-59499-0.00009-x
Dopp, J. M., Agapitov, A. V., Sinkey, C. A., Haynes, W. G., and Phillips, B. G. (2013). Sildenafil Increases Sympathetically Mediated Vascular Tone in Humans. Am. J. Hypertens, 26(6), 762–769. https://doi:10.1093/ajh/hpt018
Mitou, G., Frentzel, J., Desquesnes, A., Le Gonidec, S., AlSaati, T., Beau, I., et al. (2015). Targeting Autophagy Enhances the Anti-tumoral Action of Crizotinib in ALK-Positive Anaplastic Large Cell Lymphoma. Oncotarget, 6(30), 30149–30164. https://doi:10.18632/oncotarget.4999
Karrouchi, K., Radi, S., Ramli, Y., Taoufik, J., Mabkhot, Y., Al-aizari, F., et al. (2018). Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules, 23(1), 134. https://doi:10.3390/molecules23010134
Al-Omar, M. A. (2010). Synthesis and Antimicrobial Activity of New 5-(2-Thienyl)-1,2,4-Triazoles and 5-(2-Thienyl)-1,3,4-Oxadiazoles and Related Derivatives. Molecules, 15, 502–514. https://doi:10.3390/molecules15010502
Ran, F., Liu, Y., Zhang, D., Liu, M., and Zhao, G. (2019). Discovery of Novel Pyrazole Derivatives as Potential Anticancer Agents in MCL. Bioorg. Med. Chem. Lett. 29 (9), 1060–1064. https://doi:10.1016/j.bmcl.2019.03.005
Taher, A. T., Mostafa Sarg, M. T., El-Sayed Ali, N. R., and Hilmy Elnagdi, N. (2019). Design, Synthesis, Modeling Studies and Biological Screening of Novel Pyrazole Derivatives as Potential Analgesic and Anti-inflammatory Agents. Bioorg. Chem., 89, 103023. https://doi:10.1016/j.bioorg.2019.103023
Badavath, V. N., and Jayaprakash, V. (2020). MAO Inhibitory Activity of 4,5-dihydro-1HPyrazole Derivatives: A Platform to Design Novel Antidepressants. Front. Drug Des. Discov. 1, 45. https://doi:10.2174/9789811421563121100005
Faria, J. V., Vegi, P. F., Miguita, A. G. C., Dos Santos, M. S., Boechat, N., and Bernardino, A. M. R. (2017). Recently Reported Biological Activities of Pyrazole Compounds. Bioorg. Med. Chem. 25 (21), 5891–5903. https://doi:10.1016/j.bmc.2017.09.035
Patil, S. B. (2020). Medicinal Siganificance of Pyrazole Analogues: A Review. J. Pharm. Sci. Res. 12 (3), 402–404.
Yet, L. (2018). Privileged Structures in Drug Discovery: Medicinal Chemistry and Synthesis, in Methods and Principles in Medicinal Chemistry. London; Hoboken, NJ: John Wiley & Sons. https://doi:10.1002/9781118686263
Faisal, M., Saeed, A., Hussain, S., Dar, P., and Larik, F. A. (2019). Recent Developments in Synthetic Chemistry and Biological Activities of Pyrazole Derivatives. J. Chem. Sci. 131, 70. https://doi:10.1007/s12039-019-1646-1
Aziz, H., Zahoor, A. F., and Ahmad, S. (2020). Pyrazole Bearing Molecules as Bioactive Scaffolds: a Review. J. Chil. Chem. Soc., 65(1), 4746–4753. https://doi:10.4067/S0717-97072020000104746
Ramsay, R. R., Popovic‐Nikolic, M. R., Nikolic, K., Uliassi, E., Bolognesi, M. L. (2018). A Perspective on Multi‐target Drug Discovery and Design for Complex Diseases. Clin. Transl. Med., 7(1), 3. https://doi:10.1186/s40169-017-0181-2
Benek, O., Korabecny, J., and Soukup, O. (2020). A Perspective on Multi-Target Drugs for Alzheimer's Disease. Trends Pharmacol. Sci. 41(7), 434–445. https://doi:10.1016/j.tips.2020.04.008
He, B., Lu, C., Zheng, G., He, X., Wang, M., Chen, G., Lu, A. (2016). Combination therapeutics in complex diseases. Journal of cellular and molecular medicine, 20(12), 2231-2240. https://doi:10.1111/jcmm.12930
Ganta, R. K., Kerru, N., Maddila, S., Jonnalagadda, S. B. (2021). Advances in pyranopyrazole scaffolds’ syntheses using sustainable catalysts—a review. Molecules, 26(11), 3270. https://doi.org/10.3390/molecules26113270
Miniyar, P. B., Barmade, M. A., Mahajan, A. A. (2015). Synthesis and biological evaluation of 1-(5-(2-chloroquinolin-3-yl)-3-phenyl-1H-pyrazol-1-yl) ethanone derivatives as potential antimicrobial agents. Journal of Saudi Chemical Society, 19(6), 655-660. https://doi.org/10.1016/j.jscs.2013.12.004
Jin, M., Chen, Y., Song, W., Lian, H., Guo, H., Dong, Q. Su, J. (2020). Synthesis, characterization, and electroluminescent properties of indazole, pyrazole, and triazole/triphenylamine-based compounds. Dyes and Pigments, 173, 106912. https://doi.org/10.1016/j.dyepig.2018.07.058
Secrieru, A., O’Neill, P.M., Cristiano, M.L.S. (2020). Revisiting the Structure and Chemistry of 3(5)-Substituted Pyrazoles. Molecules., 25(1), 42. https://doi.org/10.3390/molecules25010042
Baishya, T., Gomila, R. M., Barcelo-Oliver, M., Gil, D. M., Bhattacharyya, M. K., Frontera, A. (2023). Supramolecular Assemblies in Pyridine-and Pyrazole-Based Coordination Compounds of Co (II) and Ni (II): Characterization, Hirshfeld Analysis and Theoretical Studies. Crystals, 13(2), 203. https://doi.org/10.3390/cryst13020203
Davydenko, Y. M., Vitske, V. A., Pavlenko, V. A., Haukka, M., Vynohradov, O. S., Fritsky, I. O. (2022). Synthesis, crystal structure and properties coordination polymers based on (3,5-dimethyl-1н-pyrazole-4-yl)-acetic acid. Journal of Chemistry and Technologies, 30(2), 174–183. (in Ukrainian) https://doi.org/10.15421/jchemtech.v30i2.252517
McKinnon, J. J., Jayatilaka, D., Spackman, M. A. (2007). Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chemical Communications, (37), 3814–3816. https://doi.org/10.1039/b704980c
Sheldrick G.M. (1997). SHELXS-97, Program for Crystal Structure Solution. – Göttingen, Germany.
Davydenko, Y., Pavlenko, V., Fritskiy, I., Iskenderov, I. (2010). [Synthesis and study of mononuclear copper (II) complex with 3, 5-dimethyl-1H-pyrazole]. Bulletin of Taras Shevchenko National University of Kyiv. Chemistry, (48), 9–11. (in Ukrainian).
Kokozay, V. N., Vassilyeva, O. Y., Makhankova, V. G. (2018). Direct Synthesis of Heterometallic Complexes. In Direct Synthesis of Metal Complexes. Elsevier.
Miniyar, P.B., Barmade, M.A., Mahajan, A.A. (2015). Synthesis and biological evaluation of 1-(5-(2-chloroquinolin-3-yl)-3-phenyl-1H-pyrazol-1-yl) ethanone derivatives as potential antimicrobial agents. J. Saudi Chem. Soc., 19, 655–660.
https://doi: 10.1016/j.jscs.2013.12.004
Vynohradov, O. S., Davydenko, Y. M., Pavlenko, V. O., Naumova, D. D., Fritsky, I. O., Shova, S., Prysiazhna, O. V. (2023). CuBr2 as a bromination agent of pyrazole-based ligand: synthesis of copper (II) coordination compounds by oxidative dissolution of copper powder in organic solvents. Journal of Chemistry and Technologies, 31(3), 493–506. https://doi.org/10.15421/jchemtech.v31i3.281190
Boča, R., Hvastijová, M., Kohout, J. (1994). A molecular orbital approach to coligand isomer formation. Journal of Coordination Chemistry, 33(2), 137–145. https://doi.org/10.1080/00958979408024272
Hvastijová, M., Kohout, J., Buchler, J. W., Boča, R., Kožı́šek, J., Jäger, L. (1998). Nucleophilic additions to pseudohalides in the coordination sphere of transition metal ions and coligand isomerism. Coordination chemistry reviews, 175(1), 17–42. https://doi.org/10.1016/S0010-8545(98)00208-2
Valach, F., Kohout, J., Dunaj-Jurčo, M., Hvastijová, M., & Gažo, J. (1979). Formation of a new ligand by addition of 3, 5-dimethylpyrazole to the cyanate group in a copper (II) complex: crystal and molecular structure of α-bis (1-carbamoyl-3, 5-dimethylpyrazolato) copper (II) and the physical properties of two isomeric forms. Journal of the Chemical Society, Dalton Transactions, (12), 1867–1871. https://doi.org/10.1039/DT9790001867
Szécsényi, K. M., Leovac, V. M., Češljević, V. I., Kovács, A., Pokol, G., Argay, G., Kálmán, A., Bogdanović, G.A, Jaćimović, Ž.K., Spasojević-de Bire, A. (2003). Reaction of copper (II) with 1-carboxamide-3, 5-dimethylpyrazole, 1-carboxamidine-3, 5-dimethylpyrazole, 4-acetyl-3-amino-5-methylpyrazole and 5-amino-4-carboxamide-1-phenylpyrazole. Inorganica Chimica Acta, 353, 253–262. https://doi:10.1016/S0020-1693(03)00231-7
Rosiak, D., Okuniewski, A., Chojnacki, J. (2018). Novel complexes possessing Hg–(Cl, Br, I)⋯ O= C halogen bonding and unusual Hg₂S₂ (Br/I)₄ kernel. The usefulness of τ₄′ structural parameter. Polyhedron, 146, 35–41. https://doi.org/10.1016/j.poly.2018.02.016
Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. Spackman, M. A. (2021). CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. Journal of Applied Crystallography, 54(3), 1006–1011. https://doi.org/10.1107/S1600576721002910
Van Albada, G. A., van der Horst, M. G., Bijvoets, S. M., Mutikainen, I., Turpeinen, U., Reedijk, J. (2010). New Cu(II) compounds with ligands synthesized through nucleophilic addition of pyrazoles to dicyanamide. Synthesis, crystal structures and spectroscopy. Polyhedron, 29(12), 2473–2480. https://doi.org/10.1016/j.poly.2010.05.015
Zheng, L.-L., Wang, J.-F., Wang, J., Zhou, A.-J., Liao, C.-X., Hu, S. (2020). Cu2+-Promoted Nucleophilic Addition of Pyrazole to Cyano Group. Inorganica Chimica Acta, 501, 119303. https://doi.org/10.1016/j.ica.2019.119303
Davydenko, Y. M. (2011). Coordination compounds of 3d-metals with non-chelating pyrazole ligands. Thesis for the degree of Candidate of Chemical Sciences, Taras Shevchenko National University of Kyiv: Ukraine, Kyiv.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

