MORPHOLOGY AND SIZE COMPARISON OF CRYSTALLIZED MATERIALS USING IMAGE-BASED COUNTING TECHNIQUES: CONVENTIONAL VS. SUPERCRITICAL PROCESSES
DOI:
https://doi.org/10.15421/jchemtech.v33i2.312576Keywords:
supercritical fluid; crystallization; image; particle morphology; particle size distribution.Abstract
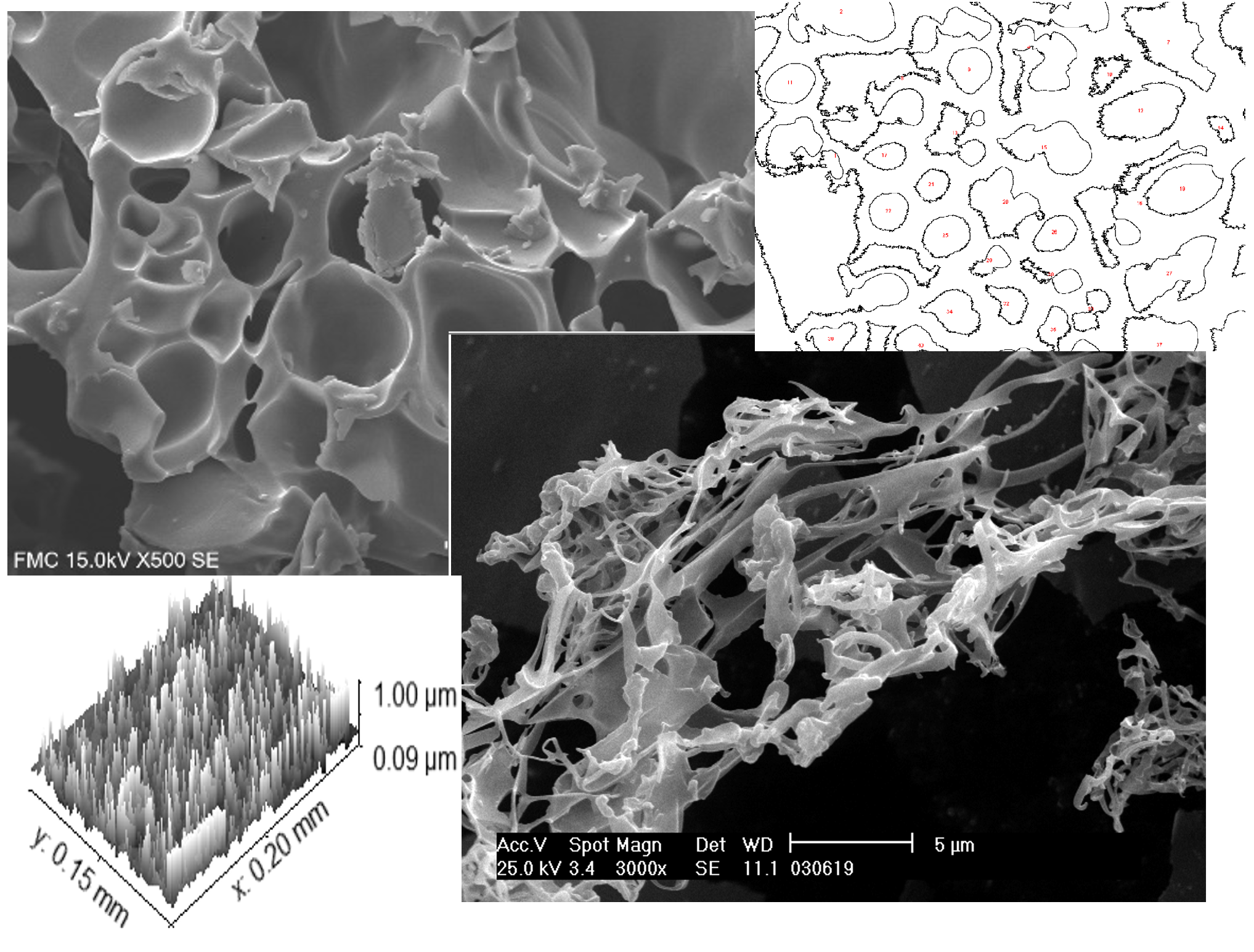
Morphological characterization of aggregates or agglomerates using image analysis is a growing topic of research in a variety of industries, including chemical, environmental, food, and pharmaceuticals. The ultimate features of agglomerates are frequently related to their size and shape distribution, and aggregate morphology can have a considerable impact on the efficiency of industrial operations as well as their health and environmental impacts. Given the significant importance of the crystallized product's shape and size, it is critical to apply technologies that allow for measurement, characterization, and quantification, image analysis in particular. This article attempts to use image analysis to determine the size and kind of aggregates analyzed, which is one of the most active study fields. It is demonstrated that, whereas conventional procedures are best suited for quantitative production jobs, supercritical processes are feasible strategies for producing such materials with recrystallized structure based on morphological range and a specified size distribution. The topic covers image analysis methodologies, aggregate characterization, and the various imaging instruments employed. Furthermore, this study demonstrates experience in managing the supercritical recrystallisation process.
References
Mok, Z. H. (2023). The effect of particle size on drug bioavailability in various parts of the body. Pharmaceutical Science Advances, 2, 100031. https://doi.org/10.1016/j.pscia.2023.100031
Bernard, P., Stelmachowski, P., Broś, P., Makowski, W., Kotarba A. (2021). Demonstration of the influence of specific surface area on reaction rate in heterogeneous catalysis, Journal of Chemical Education, 98(3), 935–940. https://doi.org/10.1021/acs.jchemed.0c01101
Sarrate, R., Ramón Ticó, J. , Miñarro, M. , Carrillo, C., Fàbregas, A. , García-Montoya, E., Pérez-Lozano, P., Suñé-Negre J. M. (2015). Modification of the morphology and particle size of pharmaceutical excipients by spray drying technique, Powder Technology, 270, 244–255. https://doi.org/10.1016/j.powtec.2014.08.021
Türk, M. (2022). Particle synthesis by rapid expansion of supercritical solutions (RESS): Current state, further perspectives and needs, Journal of Aerosol Science, 161, 105950. https://doi.org/10.1016/j.jaerosci.2021.105950.
Ksibi, H. (2024). The synergy of bioresources and supercritical fluid media: pathways to sustainable green processing. Euro-Mediterr J Environ Integr. https://doi.org/10.1007/s41207-024-00599-9
Boel, E., Koekoekx, R., Dedroog, S., Babkin, I., Vetrano, M. R., Clasen, C., and Van den Mooter, G. (2020). Unraveling Particle Formation: From Single Droplet Drying to Spray Drying and Electrospraying. Pharmaceutics, 12(7), 625. https://doi.org/10.3390/pharmaceutics12070625
Zhang, L.P., Zhao, Y.Y. (2017). Particle size distribution of tin powder produced by centrifugal atomisation using rotating cups, Powder Technology, 318, 62–67. https://doi.org/10.1016/j.powtec.2017.05.038
Maqbool, I., Noreen, S., Pervaiz, F., Ijaz, M., Farooq, I. (2019). Micro Particles: A review of recent developments, microencapsulation method, and therapeutic strategies, Global Pharmaceutical Sciences Review, IV(I), 28–39. https://doi.org/10.31703/gpsr.2019(iv-i).04.
Liu, G., Li, J., Deng, S. (2021). Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics, 13(4), 475. https://doi.org/10.3390/pharmaceutics13040475
Perrut, M. (2000). Supercritical fluid Applications: Industrial developments and economic issues, Industrial & Engineering Chemistry Research, 39(12), 4531–4535. https://doi.org/10.1021/ie000211c
Rejab, A., Ksibi, H. (2022). Particle crystallization by supercritical antisolvent processing techniques: the case of Retama raetam powder for pharmaceutical purposes, International Journal of Chemical Reactor Engineering, 21(6), 717–726. https://doi.org/10.1515/ijcre-2022-0119.
Ksibi, H., Moussa, A.B., Baccar, M. (2006). Powder Structure Transition under the Recrystallization Conditions in the RESS Process, Chemical Engineering & Technology, 29(7), 868–874. https://doi.org/10.1002/ceat.200600094
Mićić, V., Pelemiš, S. (2024). Supercritical fluids as solvents for the future, AIDASCO Reviews, 2(1), 32–40. https://doi.org/10.59783/aire.2024.45
Moussa, A.B., Ksibi, H., Baccar, M. (2008). Simulation of particles transport and coagulation during the RESS process, EPJ. Applied Physics/ the European Physical Journal Applied Physics, 43(2), 253–261. https://doi.org/10.1051/epjap:2008117
Santos, S., Puna, J., Gomes, J. (2022). A brief review of the Supercritical Antisolvent (SAS) technique for the preparation of nanocatalysts to be used in biodiesel production, Energies, 15(24), 9355. https://doi.org/10.3390/en15249355.
Lee, J. L., Chong, G. H., Ota, M., Guo, H., Smith, R. L. (2024). Solvent Replacement Strategies for Processing Pharmaceuticals and Bio-Related Compounds – A Review. Liquids, 4(2), 352–381. https://doi.org/10.3390/liquids4020018
Souiy, Z., Moussa, A.B., Ksibi, H. (2007). Numerical simulation of heat and mass transfers in a supercritical dissolution column, Chemical Engineering & Technology, 30(6), 715–720. https://doi.org/10.1002/ceat.200600371.
Ksibi H. (2011). Comparative study of numerical simulations of the RESS Process: the supercritical pure fluid Expansion, International Journal of Chemical Reactor Engineering, 9(1). https://doi.org/10.1515/1542-6580.2177.
Thakur, A.K., Kumar, R. , Kumar, V.K., Kumar, A., Kumar G. G. , Gupta K. K. (2022). A critical review on thermodynamic and hydrodynamic modeling and simulation of liquid antisolvent crystallization of pharmaceutical compounds, Journal of Molecular Liquids, 362, 119663. https://doi.org/10.1016/j.molliq.2022.119663
McGinty, J., Yazdanpanah, N., Price, C., Horst, J.H., Sefcik, J. (2020). Nucleation and crystal growth in continuous crystallization, in The Royal Society of Chemistry eBooks. https://doi.org/10.1039/9781788013581-00001
Alarcón-Apablaza, J., Navarro, P., Manzanares-Céspedes, M. C., Fuentes, R. (2024). Analysis of the Chemical Composition, Morphological Characterization, and Porosimetry of Allograft and Comparison with Xenograft for Dental Applications. International Journal of Morphology, 42(3), 698–708. https://doi.org/10.4067/s0717-95022024000300698
Provder, T. (1997). 'Challenges in particle size distribution measurement past, present and for the 21st century, Progress in Organic Coatings, 32(1–4), 143–153. https://doi.org/10.1016/s0300-9440(97)00043-x.
Subramaniam, D.N., Hitihamilage, D., Dassanayake, P. , Ahilash, N., Wijekoon, S. H. B., Sathiparan, N. (2024). Characterisation of the shape of aggregates using image analysis, International Journal of Pavement Engineering, 25(1). https://doi.org/10.1080/10298436.2024.2349905
Théodon, L., Debayle J., Coufort-Saudejaud C. (2023). Morphological characterization of aggregates and agglomerates by image analysis: A systematic literature review, Powder Technology, 430, 119033. https://doi.org/10.1016/j.powtec.2023.119033.
Bagheri, H., Hashemipour, H., Ghalandari, V., Ghader, S. (2021). Numerical solution of particle size distribution equation: Rapid expansion of supercritical solution (RESS) process, Particuology, 57, 201–213. https://doi.org/10.1016/j.partic.2020.12.011.
Kumari, N.R., Rana, N.N. (2015). Particle Size and Shape Analysis using Imagej with Customized Tools for Segmentation of Particles, International Journal of Engineering Research and Technology, V4(11). https://doi.org/10.17577/ijertv4is110211.
Clercq, S., Crampon, C., Badens, E. (2024). Atypical crystal growth within the Supercritical Antisolvent process: experimental and molecular modeling approach with Sodium Bicarbonate, The Journal of Supercritical Fluids, 207, 106188. https://doi.org/10.1016/j.supflu.2024.106188
Carretier, E., Badens, E., Guichardon, P., Boutin, O., Charbit, G. (2002). Hydrodynamics of Supercritical Antisolvent Precipitation: Characterization and influence on particle morphology, Industrial & Engineering Chemistry Research, 42(2), 331–338. https://doi.org/10.1021/ie020439v.
Chen, C., Yan, X., Wu Y. , Zhang, X., Liu, S., Zhang, F., Sun, X., Zhu, Q., Zheng, L., Zhang, J., Xing, X., Wu, Z., Han B. (2023). Oxidation of metallic Cu by supercritical CO2 and control synthesis of amorphous nano-metal catalysts for CO2 electroreduction, Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-36721-8
Kondoh, E., Kato, H. (2002). Characteristics of copper deposition in a supercritical CO2 fluid, Microelectronic Engineering, 64(1–4), 495–499. https://doi.org/10.1016/s0167-9317(02)00826-2
Ivanović M, Knez Ž, Leitgeb M. (2023) Influence of Supercritical Carbon Dioxide on the Activity and Conformational Changes of α-Amylase, Lipase, and Peroxidase in the Solid State Using White Wheat Flour as an Example. Foods. 16;12(24), 4499. doi: 10.3390/foods12244499.
Ksibi, H. (2024b). Supercritical Fluid for Retama Raetam Porous Film Production: A Strategy for Advancing Drug Dosage. Current Trends in Biotechnology and Pharmacy, 18(3), 1904–1912. https://doi.org/10.5530/ctbp.2024.3.36
Chakravarty, P., Famili, A., Nagapudi K., Al-Sayah M.A. (2019). Using supercritical fluid technology as a green alternative during the preparation of drug delivery systems. Pharmaceutics, 11(12), 629. https://doi.org/10.3390/pharmaceutics11120629
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

