INVESTІGATІON OF CONDІTІONS FOR OBTAІNІNG AG3SBS4 NANOPARTІCLES ІN VARІOUS ORGANІC SOLVENTS
DOI:
https://doi.org/10.15421/jchemtech.v33i2.313814Keywords:
Keywords nanocrystals, ethylene glycol polyethylene glycol, dimethylformamide triethanolamine, n-heptane.Abstract
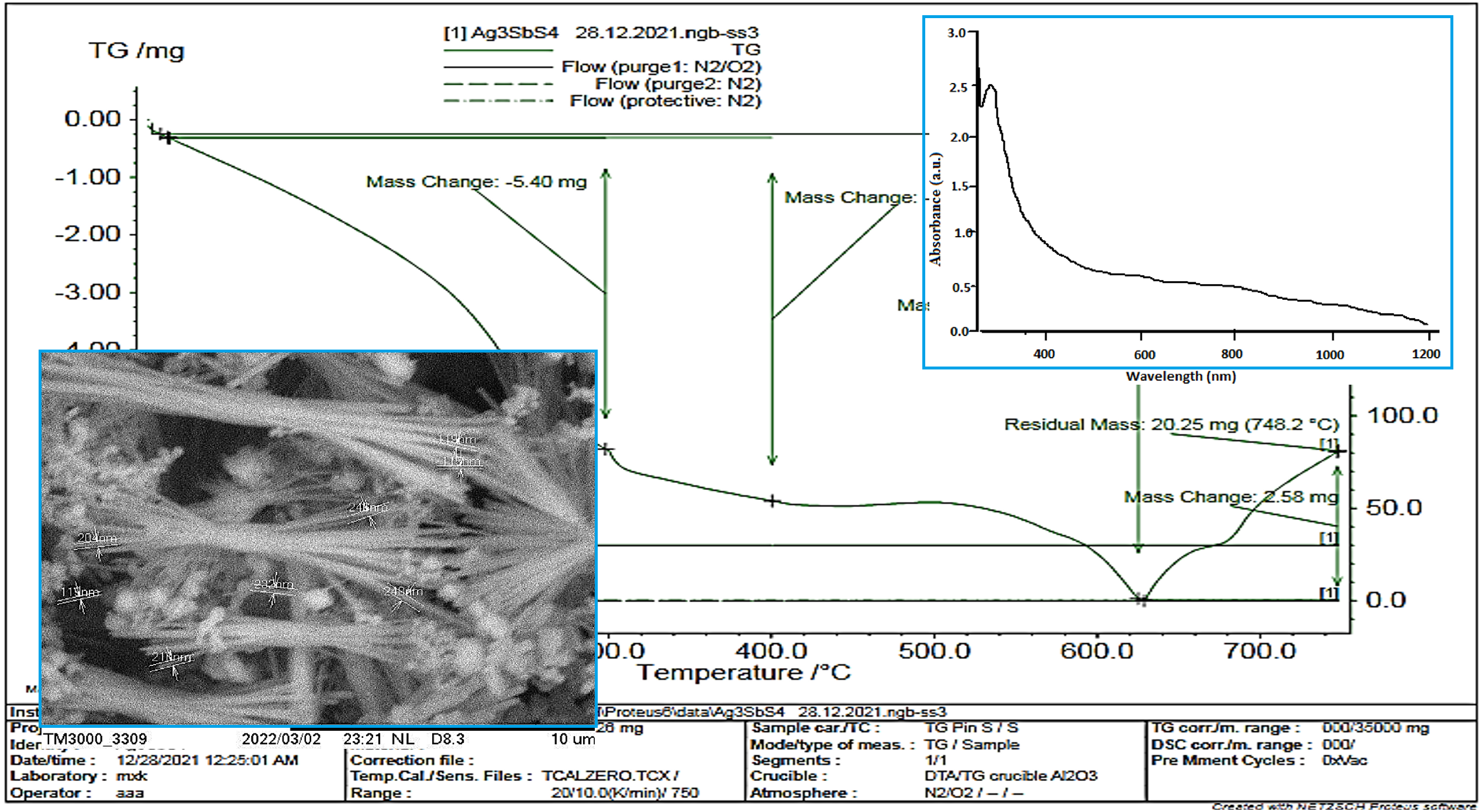
Ag3SbS4-nanocrystals were synthesized by solvothermal method in various member solvents (ethylene glycol+polyethylene glycol) and (dimethylformamide+triethenolamine). Optimum conditions for obtaining nanoparticles were determined (molar ratios of the amount of components, volume ratio of the solvent, temperature, time, etc.). The crystal structure, morphology, nano-size and chemical composition of the obtained silver thiostibiate nanostructure were studied using thermogravimetric (TG), X-ray powder diffractometer (XRD), energy dispersion spectroscopy (eds) and transmission electron microscopy (TEM). Using EDX and chemical analysis, the composition was found to correspond to the formula Ag3SbS4. The results of the TEM analysis showed that the synthesized Ag3SbS4 nanowires have diameters ranging from 150 to 200 nm, while their lengths can reach several micrometers. The dispersion solution of Ag3SbS4 nanoparticles was prepared in n-heptane, and the forbidden band width was determined from the absorption spectrum of the solution.
References
Biagioni, C., Zaccarini, F., Roth, P., Bindi, L. (2020). Progress in the knowledge of ‘ruby silvers’: New structural and chemical data of pyrostilpnite, Ag3SbS3. Mineralogical Magazine, 84, 463–467 https://doi.org/10.1180/mgm.2020.37
Keighin, C.W., Honea, R.M. (1969). The system Ag-Sb-S from 600°C to 200°C. Mineral. Deposita, 4, 153–171. https://doi.org/10.1007/BF00208050
Zhang, L., Zhu, C., Chen, T. (2021). Solution processed AgSbS2 film for efficient planar heterojunction solar cells. Applied Physics Letters, 119(15), 15 https://doi.org/10.1063/5.0064802
Gusain, M., Rawat, P., Nagarajan, R. (2014). Soft chemical synthesis of Ag3SbS3 with efficient and recyclable visible light photocatalytic properties. Materials Research Bulletin, 60, 872–875. https://doi.org/10.1016/j.materresbull.2014.09.084
Satra, J., Ghorui, U. K., Mondal, Р., Bhadu, G. R. (2020). One pot solvent assisted syntheses of Ag3SbS3 nanocrystals and exploring their phase dependent electrochemical behavior toward oxygen reduction reaction and visible light induced methanol oxidation reaction. Dalton Trans., 49, 9464–9479 http:/doi.org/10.1016/j.mssp.2021.106167
Zhong, J., Hu, J., Cai, W., Yang, F. (2010). Biomolecule-assisted synthesis of Ag3SbS3 nanorods. Journal of Alloys and Compounds, 501(1), 15–19. https://doi.org/10.1016/j.jallcom.2010.04.070
Gomez, D. T., Henry, J., Mohanraj, K., Sivakumar, G. (2016). AgSbS2 and Ag3SbS3 absorber materials for photovoltaic applications. Materials Chemistry and Physics, 181, 415–421. DOI:10.1016/j.matchemphys.2016.06.077
Tubtimtae, A., Huang, Ch.-L., Shi, J.-B., Lee, M.-W (2016). Ag3SbS3 thin films formed by annealing hydrothermally synthesized Ag3SbS3 nanoparticles. Materials Letters, 177, 58–60. doi: 10.1016/j.matlet.2016.04.165
Ho, Y.-R., Lee, M.-W. (2013). AgSbS2 semiconductor-sensitized solar cells. Electrochemistry Communications, 26, 48–51. https://doi.org/10.1016/j.elecom.2012.10.003
Boon-on, P., Chen, P.-H., Lee, M-W. (2022). AgSbSe2 nanoparticles: A solarabsorber material with an optimal Shockley-Queisser band gap. Materials Letters, 309, 131412. https://doi.org/10.1016/j.matlet.2021.131412
Chalapathi, U. Reddy, A. S., Prasad, P. R. (2023). Two-stage-processed AgSbS2 films for thin-film solar cells/ Materials Science in Semiconductor Processing, 168, 107821 https://doi.org/10.1016/j.mssp.2023.107821
Hosni, N., Bouaniza, N., Selmi, W., Assili, K. Maghraoui-Meherzi, H. (2019). Synthesis and physico-chemical investigations of AgSbS2 thin films using Doehlert design and under DFT framework. Journal of Alloys and Compounds, 778, 913–923.
Medina-Montes, M.I., Baldenegro-Pérez, L.A., Morales-Luna, M., Sánchez, T.G., Santos-Cruz, D., Mayén-Hernández, S.A. (2022). Physical properties of photoconductive Ag-Sb-S thin films prepared by thermal evaporation. Materials Science in Semiconductor Processing, 137, 106167 https://doi.org/10.1016/j.mssp.2021.106167
Chalapathi, Ch., Hemalatha, A. S., Dhanalakshmi, R., Reddy, G.S., Divya, V. Gonuguntla, S. Sangaraju, A., Ayman, İ., Ghfar, P., Rosaiah, S., Youngsuk, S.Y. (2024). Park Impact of stacking sequence and sulfurization duration on the growth of pyrargyrite Ag3SbS3 absorber layers. Journal of Solid State Chemistry, 338, 124832
Oubakalla, M. Mouhcine, B., Khalid, F., Nejmi, Y. (2023). The Ag3SbS3 thin film combining super-capacitive and absorptive behaviors: elaboration, characterization and DFT study. Applied Physics A, 130(1) doi:10.1007/s00339-023-07183-y
Zhiping, J., Xue, C. L. Y., Huanq, D., Persson, C. (2023). First-principles prediction on Ag3SbS4 as a photovoltaic absorber. Journal of Physics and Chemistry of Solids, 183, 111655. https://doi.org/10.1016/j.jpcs.2023.111655
Aspiala, M., Tesfaye, F., Taskine, P. (2016). Thermodynamic study in the Ag–Sb–S system by the EMF method. The Journal of Chemical Thermodynamics 98, 361–366. https://doi.org/10.1016/j.jct.2016.03.009
Dimitrov, R. Boyanov, B. (2004). Oxidation of Metal Sulphides and Determination of Characteristic Temperatures by DTA and TG. Journal of Thermal Analysis and Calorimetry, 61(1), 181–189 https://doi.org/10.1023/A:1010181112713
Abbasov, A.D., Mamedova, G. A. (2025). Synthesis of Clinoptilolite Zeolite and Investigation the Influence of Various Factors on the Crystallization Process in Natural System Obsidian-Halloysite. Theoretical Foundations of Chemical Engineering, 58, 1206–1217 https://doi.org/10.1134/S0040579524600347
Aliyeva, S. H., Rzayeva, A. B. (2024). Synthesis of copper thiostibiate nano compound by solvothermal method. Journal of Chemistry and Technologies, 33(2), 304–311. https://doi.org/10.15421/jchemtech.v32i2.299093
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

