NIR-LUMINESCENCE OF YTTERBIUM IONS IN ISOMERIC TETRAPHENYLPORPHYRIN MODIFIED EDTA COMPLEXES
DOI:
https://doi.org/10.15421/jchemtech.v33i1.315032Keywords:
porphyrins, lanthanides, luminescence, energy transferAbstract
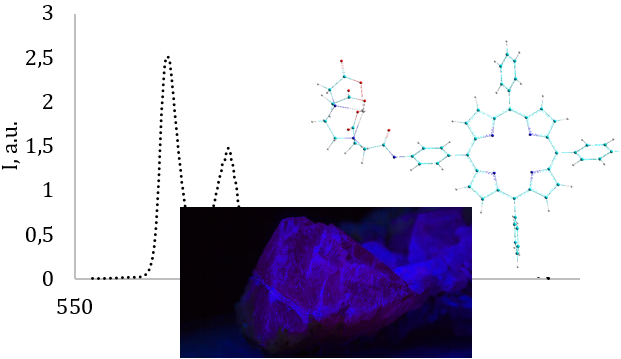
New ytterbium complexes were synthesized on the base of tetraphenylporphyrin modified with ethylenediaminetetraacetic acid at the ortho- and para-positions of only one phenyl ring. Such modification avoids the problem of lability of traditional lanthanide-porphyrin core-coordinated complexes. A new selective synthetic route for the mono para-nitro-derivative of tetraphenylporphyrin (the main precursor of the target compound) has been proposed. 4f-Luminescence in the near-infrared range as well as non-quenched molecular fluorescence of porphyrin, are observed in all synthesized complexes, which makes these complexes dual-emissive. Since the obtained results show that both Yb-porphyrin isomers have no changes in terms of fluorescence effectiveness in comparison to their corresponding ditopic porphyrins, it proves the absence of ISC and ISD acceleration in these systems. It was also found that the 4f-luminescence intensity of the ortho-isomer is higher compared to the para-isomer. This is due to changes in the spatial structure, leading to the edta-Yb fragment being closer to the porphyrin core. Additional experiment of luminescence quenching by strong paramagnetic ion was performed. When the copper (II) acetate was added to the modified porphyrin isomers, a significant difference in the efficiency of luminescence quenching was observed as the result of non-core interaction; thus, the luminescence intensity decreased more for the ortho-isomer than for the para-isomer. This also proves that the efficiency of interaction between porphyrin and the peripheral substituent is very sensitive to the distance changes between them. Enhancing of 4f-luminescence effectiveness can be explained simultaneously by both decreasing the donor-acceptor distance and the absence of notable ISD acceleration which is typical for core-coordinated compounds.
References
Puccini, A., Liu, N., Hemmer, E. (2024). Praseodymium-Doped Nanoparticles: Candidates for Near-Infrared-II Double- and Single-Band Nanothermometry. ACS Materials Letters, 6(4), 1327–1337. https://doi.org/10.1021/acsmaterialslett.3c01554
Malhotra, K., Hrovat, D., Kumar, B., Qu, G., Houten, J. V., Ahmed, R., Piunno, P. a. E., Gunning, P. T., Krull, U. J. (2023). Lanthanide-Doped Upconversion Nanoparticles: Exploring A Treasure Trove of NIR-Mediated Emerging Applications. ACS Applied Materials & Interfaces, 15(2), 2499–2528. http://dx.doi.org/10.1021/acsami.2c12370
Li, J., Li, Y., Selishchev, D., Zhang, G. (2024). Near-infrared responsive photocatalysts for environmental remediation and energy conversion: A review. Chemosphere, 367, 143599. https://doi.org/10.1016/j.chemosphere.2024.143599
Liu, Y., Wang, H., Qu, S. (2025). Review on near-infrared absorbing/emissive carbon dots: From preparation to multi-functional application. Chinese Chemical Letters, 36(5), 110618. https://doi.org/10.1016/j.cclet.2024.110618
Khan, M. A., Asadi, H., Zhang, L., Qazani, M. R. C., Oladazimi, S., Loo, C. K., Lim, C. P., Nahavandi, S. (2024). Application of artificial intelligence in cognitive load analysis using functional near-infrared spectroscopy: A systematic review. Expert Systems with Applications, 249, 123717. https://doi.org/10.1016/j.eswa.2024.123717
Du, Y., Ni, S., Ma, Q., Song, X., Yang, H. (2023). Engineering NIR-II luminescent lanthanide nanoprobes for imaging brain diseases in vivo. Coordination Chemistry Reviews, 496, 215401. https://doi.org/10.1016/j.ccr.2023.215401
Luo, X., Zhang, C., Yu, Z., Wen, S., Xian, Y. (2023). Recent advances in responsive lanthanide-doped luminescence nanoprobes in the near-infrared-II window. TrAC Trends in Analytical Chemistry, 169, 117368. https://doi.org/10.1016/j.trac.2023.117368
Falsaperla, R., Leone, G., Giallongo, A., Giacchi, V., Lombardo, G., Polizzi, A., Romano, C., Ruggieri, M. (2025). Near-infrared spectroscopy (NIRS) as a tool to prevent cerebral desaturation in newborns with bradycardia events: A systematic review. Pediatrics & Neonatology, 66(2), 94–101. https://doi.org/10.1016/j.pedneo.2024.07.011
Gan, Y., Ying, J., Qiu, X., You, S., Zhang, T., Ruan, T., Zhou, R., Ye, Y., Yue, Y., Zhang, L., Mu, D. (2024). Value of near-infrared spectroscopy in evaluating the risk of neonatal necrotizing enterocolitis: A systematic review and meta-analysis. Early Human Development, 195, 106083. https://doi.org/10.1016/j.earlhumdev.2024.106083
Wang, T., Wang, S., Liu, Z., He, Z., Yu, P., Zhao, M., Zhang, H., Lu, L., Wang, Z., Wang, Z., Zhang, W., Fan, Y., Sun, C., Zhao, D., Liu, W., Bünzli, J.-C. G., Zhang, F. (2021). A hybrid erbium(III)–bacteriochlorin near-infrared probe for multiplexed biomedical imaging. Nature Materials, 20(11), 1571–1578. https://doi.org/10.1038/s41563-021-01063-7
Altmann, A., Eden, M., Hüttmann, G., Schell, C., Rahmanzadeh, R. (2023). Porphyrin-based sensor films for monitoring food spoilage. Food Packaging and Shelf Life, 38, 101105. https://doi.org/10.1016/j.fpsl.2023.101105
Li, L.-L., Diau, E. W.-G. (2013). Porphyrin-sensitized solar cells. Chem. Soc. Rev., 42(1), 291–304. https://doi.org/10.1039/c2cs35257e
Duc La, D., Khong, H.M., Nguyen, X.Q., Dang, T.-D., Bui, X.T., Nguyen, M. K., Ngo, H. H., Nguyen, D. D. (2024). A review on advances in graphene and porphyrin-based electrochemical sensors for pollutant detection. Sustainable Chemistry One World, 3, 100017. https://doi.org/10.1016/j.scowo.2024.100017
Atal, K., Phageria, U., Kumari, S., Dhayal, Y., Bugalia, S. (2024). A review on designing and synthesis of lanthanide based macrocyclic complexes and their potential applications. Inorganica Chimica Acta, 561, 121857. https://doi.org/10.1016/j.ica.2023.121857
Li, L., Wang, Z., Zhao, L., Liu, H., Li, Y. (2025). Lanthanide-based photocatalysts for CO2 conversion: Are they a better choice for realizing sustainability? Coordination Chemistry Reviews, 522, 216223. https://doi.org/10.1016/j.ccr.2024.216223
Hu, J.-Y., Ning, Y., Meng, Y.-S., Zhang, J., Wu, Z.-Y., Gao, S., Zhang, J.-L. (2017). Highly near-IR emissive ytterbium(iii) complexes with unprecedented quantum yields. Chemical Science, 8, (4), 2702-2709. http://dx.doi.org/10.1039/c6sc05021b
Kuznetsova, R. T., Ermolina, E. G., Gadirov, R. M., Mayer, G. V., Semenishin, N. N., Rusakova, N. V., Korovin, Y. V. (2010). Luminescence of metal complexes of chelate-substituted tetraphenylporphyrin. High Energy Chem., 44(2), 134–138. https://doi.org/10.1134/s0018143910020098
Rusakova, N., Semenishyn, N., Korovin, Y. (2010). Heteronuclear lanthanide-containing complexes on the base of modified porphyrins and their luminescent properties. J. Porphyrins Phthalocyanines, 14(2), 166–169. https://doi.org/10.1142/S1088424610001817
Shin K., Y. D.-H. (1999). Facile synthesis of porphyrin-EDTA conjugate and porphyrin-DTPA conjugate. J. Korean Chem. Soc., 43(6), 611–613.
Hofmann, B., Bogdanov Jr., A., Marecos, E., Ebert, W., Semmler, W., Weissleder, R. (1999). Mechanism of gadophrin-2 accumulation in tumor necrosis. Journal of Magnetic Resonance Imaging, 9(2), 336–341. https://doi.org/10.1002/(SICI)1522-2586(199902)9:2<336::AID-JMRI28>3.0.CO;2-3
Semenishyn, N. N., Smola, S. S., Rusakova, M. Y., Rusakova, N. V. (2022). 4f-Luminescence of 3d-4f heteronuclear porphyrin complexes. Journal of Chemistry and Technologies, 30(3), 363–369. https://doi.org/10.15421/jchemtech.v30i3.261538
Semenishyn, N. N., Smola, S. S., Rusakova, N. V., Martynov, A. G., Birin, K. P., Gorbunova, Y. G., Tsivadze, A. Y. (2018). Infrared 4f-Luminescence of Erbium(III) Complexes with Tetrapyrrole Ligands. Macroheterocycles, 11(3), 262–268. https://doi.org/10.6060/mhc180691r
Rao, T. A., Maiya, B. G. (1994). Spectroscopic, redox and emission properties of 2-nitro-substituted free base- and metallo-tetra-aryl porphyrins. Polyhedron, 13(12), 1863–1873. http://dx.doi.org/10.1016/0277-5387(94)80009-X
Uemori, Y., Kitamura, A., Munakata, H., Imai, H., Nakagawa, S. (2001). Syntheses and characterization of porphyrins and their zinc complexes appending a multi-dentate ligand. Inorganica Chimica Acta, 325(1), 29–35. https://doi.org/10.1016/S0020-1693(01)00630-2
Kruper, W. J., Jr., Chamberlin, T. A., Kochanny, M. (1989). Regiospecific aryl nitration of meso-substituted tetraarylporphyrins: a simple route to bifunctional porphyrins. The Journal of Organic Chemistry, 54(11), 2753–2756. https://doi.org/10.1021/jo00272a057
Andres, J., Chauvin, A.-S. (2013). Energy transfer in coumarin-sensitised lanthanide luminescence: investigation of the nature of the sensitiser and its distance to the lanthanide ion. Physical Chemistry Chemical Physics, 15(38), 15981–15994. https://doi.org/10.1039/C3CP52279B
Horrocks, W. D., Bolender, J. P., Smith, W. D., Supkowski, R. M. (1997). Photosensitized Near Infrared Luminescence of Ytterbium(III) in Proteins and Complexes Occurs via an Internal Redox Process. Journal of the American Chemical Society, 119(25), 5972–5973. https://doi.org/10.1021/ja964421l
Abusaleh, A., Mearest, C. F. (1984). Excitation and de-excitation processes in lanthanide chelates bearing aromatic sidechains. Photochemistry and Photobiology, 39(s1), 763–769. https://doi.org/10.1111/j.1751-1097.1984.tb08856.x
Gastilovich, E.A., Korol’kova, N.V., Serov, S.A., Klimenko, V.G., Nurmukhametov, R. N. (2008). Effect of the vibrational pattern of out-of-plane vibrational modes on vibronically induced spin-orbit coupling between ππ* states involved in nonradiative intersystem crossing transitions. Optics and Spectroscopy, 105(2), 208–216. https://doi.org/10.1134/S0030400X08080080
Rosa, A., Ricciardi, G., Baerends, E. J. (2006). Synergism of Porphyrin-Core Saddling and Twisting of meso-Aryl Substituents. The Journal of Physical Chemistry A, 110 (15), 5180–5190. https://doi.org/10.1021/jp060931i
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

