REGIOSELECTIVITY OF HALO- AND CHALCOGEN-INDUCED CYCLIZATION OF DIALLYLQUINAZOLIN-4-ONE
DOI:
https://doi.org/10.15421/jchemtech.v32i4.316035Keywords:
3-allyl-2-allylthioquinazolin-4-one, thiazolo[3,2-a]quinazoline, electrophilic intramolecular cyclization, regioselectivity, organohalogen compounds, organochalcogen compoundsAbstract
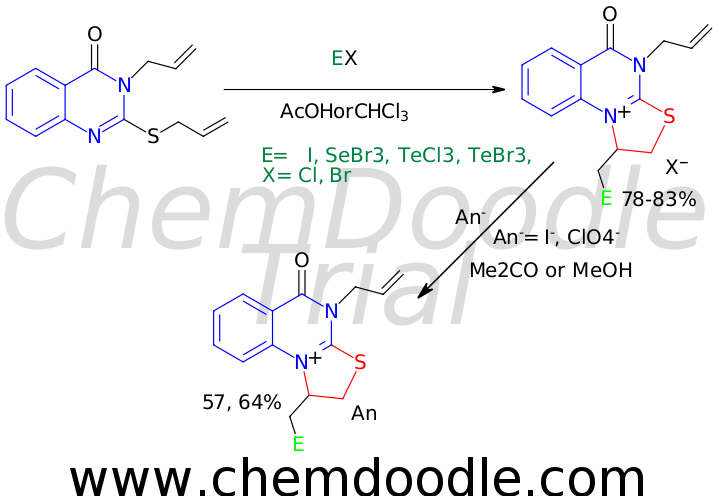
Objective. There is significant interest among medicinal chemists in compounds containing the quinazoline fragment due to their broad spectrum of biological activity and the need to develop new synthetic methods for these systems. Special attention is given to alkenyl and alkynyl derivatives of quinazolines, which serve as convenient models for studying electrophilic heterocyclization reactions. This study examines the effect of the electrophilic reagent’s nature on the regiochemistry of electrophilic intramolecular heterocyclization of 3-allyl-2-allylthioquinazolin-4-one. Methods. 1H and 13C NMR spectroscopy. Results. Experimental data indicate that the interaction of 3-allyl-2-allylthioquinazolin-4-one with hybrid halogen (iodine bromide) and the tetrahalides of selenium and tellurium leads to the regioselective formation of angular monohalides of 4-allyl-5-oxo-1,2,4,5-tetrahydrothiazolo[3,2-a]quinazolinium. It was noted that an excess of the electrophilic reagent does not affect the direction of halo- and chalcogen-induced heterocyclization. The interaction of 4-allyl-1-(iodomethyl)-5-oxo-1,2,4,5-tetrahydrothiazolo[3,2-a]quinazolin-10-ium bromide with potassium iodide and sodium perchlorate yielded the corresponding thiazolo[3,2-a]quinazolinium salts, confirming the stability of the organic cation in ion exchange reactions. Conclusions. This study investigated the regioselectivity of the electrophilic heteroannulation reaction of 3-allyl-2-allylthioquinazolin-4-one under the influence of halo- and chalcogen-containing electrophiles, resulting in monohalide salts of tetrahydrothiazolo[3,2-a]quinazolin-10-ium containing an allyl fragment at the 4-position of the thiazoloquinazoline.
References
Mass, E. B., Duarte, G. V., Russowsky, D. (2021). The Quinazoline-Chalcone and Quinazolinone-Chalcone Hybrids: A Promising Combination for Biological Activity. Mini-Rev. Med. Chem., 197(2), 186–203. https://doi.org/10.2174/1389557520666200730160325
Kut, D., Kut, M., Komarovska-Porokhnyavets, O., Kurka, M., Onysko, M., Lubenets, V. (2024). Antimicrobial Activity of Halogen- and Chalcogen-Functionalized Thiazoloquinazolines. Lett. Drug. Des. Discov., 21(13), 2490–2496. https://doi.org/10.2174/1570180820666230726160348
Pylypenko, O.O., Sviatenko, L.K., Shabelnyk, K.P., Kovalenko, S.I., Okovytyy, S.I. (2024). Synthesis and hydrolytic decomposition of 2-hetaryl[1,2,4]triazolo[1,5-c]quinazolines: DFT study. Struct. Chem., 35(1), 97–104. https://doi.org/10.1007/s11224-023-02251-8
Antypenko, L., Antypenko, O., Karnaukh, I.,
Rebets, O., Kovalenko, S., Arisawa, M. (2023). 5,6-Dihydrotetrazolo[1,5-c]quinazolines: Toxicity prediction, synthesis, antimicrobial activity, molecular docking, and perspectives. Arch. Pharm., 356(6), 2300029. https://doi.org/10.1002/ardp.202300029
Pantyo, V.V., Haleha, O.V., Kut, D.Z., Kut, M.M., Onysko, M.Y., Danko, E.M., Koval, G.M., Pantyo, V.I., Haza, K.V., Bulyna, T.B. (2024). The effect of low-intensity laser radiation on the sensitivity of Staphylococcus aureus to some halogen-containing azaheterocycles. Regul. Mech. Biosyst., 15(2), 230–234. https://doi.org/10.15421/022434
Stavytskyi, V., Antypenko, O., Devinyak, O., Voskoboinik, O., Kovalenko, S. (2022). QSAR and pharmacophore models for screening anti-inflammatory activity among substituted (pyrrolо[1,2-a][1,2,4]triazino[2,3-c]quinazoline-5a-yl)carboxylic acids. J. Pharm. Res., 26(5), 1420–1431.
Mir, S.A., Nayak, B. (2023). Exploring binding stability of hydroxy-3-(4-hydroxyphenyl)-5-(4-nitrophenyl)-5,5a,7,8,9,9a-hexahydrothiazolo[2,3-b]quinazolin-6-one with T790M/L858R EGFR-TKD. J. Biomol. Struct. Dyn., 41, 3702–3716. https://doi.org/10.1080/07391102.2022.2053748
Mir, S. A., Mohanta, P. P., Meher, R. K., Baitharu, I., Raval, M. K., Behera, A. K., Nayak, B. (2022). Structural insights into conformational stability and binding of thiazolo-[2,3-b] quinazolinone derivatives with EGFR-TKD and in-vitro study. Saudi J. Biol. Sci., 29, 103478. https://doi.org/10.1016/j.sjbs.2022.103478
Keshari, K. A., Singh, A. K., Raj, V., Rai, A., Trivedi, P., Ghosh, B., Kumar, U., Rawat, A., Kumar, D., Saha, S. (2017). p-TSA-promoted syntheses of 5H-benzo[h] thiazolo[2,3-b]quinazoline and indeno[1,2-d]thiazolo[3,2-a]pyrimidine analogs: molecular modeling and in vitro antitumor activity against hepatocellular carcinoma. Drug Des. Dev. Ther., 11, 1623–1642. doi: 10.2147/DDDT.S136692.
Dawood, D. H., Jasass, R. S., Amin, M. M., Farghaly, T. A., Abbas, E. M. H. (2017). Synthesis of Some New Azoloazines with Potent Anti-inflammatory and Analgesic Activity. J. Heterocycl. Chem., 54, 1578–1589. https://doi.org/10.1002/jhet.2746
Panneerselvam, T., Arumugam, S., Selvaraj, K., Sankarganesh, A., Indhumathy, M., Sivakumar, A. (2017). Design, Network Analysis, In silico Modeling and Synthesis of Biologically Active Thiazolo Quinazoline Scaffolds as Anti-tubercular Agent. Curr. Chem. Biol., 11, 140–149. https://doi.org/10.2174/2212796811666170615131140
Mir, S. A., Dash, G. C., Meher, R. K., Mohanta, P.P., Chopdar, K. S., Mohapatra, P. K., Baitharu, I., Behera, A. K., Raval, M.K., Nayak, B. (2022). In Silico and In Vitro Evaluations of Fluorophoric Thiazolo-[2,3-b]quinazolinones as Anti-cancer Agents Targeting EGFR-TKD. Appl. Biochem. Biotechnol., 194, 4292–4318. https://doi.org/10.1007/s12010-022-03893-w
Kut, M. M., Onysko, M. Y. (2021). Synthesis of functionalized azolo(azino)quinazolines by electrophilic cyclization (microreview). Chem. Heterocycl. Comp., 57(5), 528–530. https://doi.org/10.1007/s10593-021-02937-z
Kut, D. Zh., Kut, М. М., Ostapchuk, Є. М., Onysko, М. Yu. (2023). [Regio- and stereo-selective halogen-induced cyclization of terminal alkynyl thioethers of 3-phenylquinazoline-4-one]. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (6), 124–128 (in Ukrainian). http://dx.doi.org/10.32434/0321-4095-2023-151-6-124-128
Zborovskyi, Y.L., Orysik, V.V., Dobosh, O.O., Hrypak, S.M., Nesterenko, O.M., Stanynets V.I. (2002). [Synthesis of thiazolo- and thiazino[3,2-a]quinazoline derivatives]. Ukrainskii khimicheskii zhurnal – Ukrainian Chemistry Journal, 68(12), 95–99 (in Ukrainian).
Kut, D., Kut, M., Svalyavin, O., Onysko, M., Lendel, V. (2022). Halogenoheterocyclization of terminal and internal 2-allylthio-3-methyl(phenyl)-7-trifluoromethylquinazolin-4-ones. Phosphorus Sulfur Silicon Relat. Elem., 197(12), 1255–1262. https://doi.org/10.1080/10426507.2022.2085275
Vaskevych, R.I., Savinchuk, N.O., Vaskevych, A.I., Rusanov, E. B., Bylina, D. V. Kyrylchuk, A.A., Vovk, M.V. (2022). Proton- and halogen-induced cyclizations of 2-(3-butenyl)quinazolin-4(3H)-ones in the synthesis of pyrrolo[2,1-b]- and pyrrolo[1,2-а]quinazolinone derivatives. J. Heterocycl. Chem., 60(3), 431–448. https://doi.org/10.1002/jhet.4598
Vaskevych, A. I., Savinchuk, N. O., Vaskevych, R. I., Rusanov, E. B., Grygorenko, O. O., Vovk, M. V. (2021). The PIFA-initiated oxidative cyclization of 2-(3-butenyl)quinazolin- 4(3H)-ones - an efficient approach to 1-(hydroxymethyl)- 2,3-dihydropyrrolo[1,2-a]quinazolin-5(1H)-ones. Beilstein J. Org. Chem., 17, 2787–2794. https://doi.org/10.3762/bjoc.17.189
Vaskevych, A. I., Savinchuk, N. O., Vaskevych, R. I., Rusanov, E. B., Vovk, M. V. (2022). Chalcogenation/pyrrolo(pyrido)annulation of 2-(3-butenyl)quinazolin-4(3H)-ones by arylsulfenyl(selenyl) chlorides. Tetrahedron, 111, 132722. https://doi.org/10.1016/j.tet.2022.132722
Kut, D. Z., Kut, M. M., Ostapchuk, E. M., Onysko, M. Yu, Onys’ko, P. P., Lendel, V. G. (2024). Versatile synthesis of 2-functionalized dihydrothiazolo[2,3-b]quinazolines through regioselective electrophilic intramolecular heterocyclization of 3-alkenyl-2-thioxoquinazolin-4-ones. Phosphorus Sulfur Silicon Relat. Elem., Accepted. https://doi.org/10.1080/10426507.2024.2416210
Gurnani, C., Jura, M., Levason, W., Ratnani, R., Reid, G., Webster, M. (2009). Preparation and structures of tellurium(IV) halide complexes with thioether coordination. Dalton Trans., (21), 4122–4128. https://doi.org/10.1039/B902771H
Kut, D. Zh., Kut, М. М., Onysko, М. Yu., Lendel, V.G. (2021). [Electrophilic cyclization of propargyl thioethers of 3-methyl(phenyl)-2-(prop-2-yn- 1-ylthio)-7-(trifluoromethyl)quinazolin-4(3H)-ones by tellurium tetrahalides]. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (6), 124–128 (in Ukrainian). http://dx.doi.org/10.32434/0321-4095-2021-139-6-40-44
Slivka, M., Korol, N., Pantyo, V., Baumer, V., Lendel, V. (2017). Regio- and stereoselective synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium salts via electrophilic heterocyclization of 3-S-propargylthio-4Н-1,2,4-triazoles and their antimicrobial activity. Heterocycl Commun., 23(2), 109–113. https://doi.org/10.1515/hc-2016-0233
Slivka, M., Fizer, M., Mariychuk, R., Ostafin, M., Moyzesh, O., Koval, G., Holovko-Kamoshenkova, O., Rusyn, I., Lendel, V. (2024). Synthesis and Antimicrobial Activity of Functional Derivatives of thiazolo[ 2,3-c][1,2,4]triazoles. Lett. Drug. Des. Discov., 19(9), 791–799. https://doi.org/10.2174/1570180819666220110145659
Paegle, Е., Belyakov, S., Petrova, M., Liepinsh, E., Arsenyan, P. (2015). Cyclization of Diaryl(hetaryl)alkynes under Selenobromination Conditions: Regioselectivity and Mechanistic Studies. Eur. J. Org. Chem., 2015(20), 4389–4399. https://doi.org/10.1002/ejoc.201500431
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

