CRYSTAL GROWTH AND OPTICAL PROPERTIES ANIZOTROPY OF α-NiSO4×6H2O
DOI:
https://doi.org/10.15421/jchemtech.v32i4.316209Keywords:
growth from solutions, optical transmission spectra, thermal analysis, band gap, X-ray diffractionAbstract
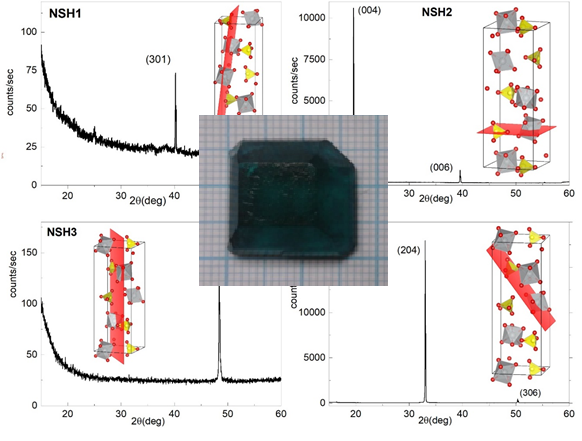
α-NiSO4×6H2O single crystal was grown by slow evaporation technique and characterized by X-ray powder diffraction, differential thermal analysis, UV-Vis–NIR, FTIR, and atomic absorption spectroscopy methods. Combination of differential thermal analysis, X-ray powder diffraction and atomic absorption spectroscopy indicates a purity and phase homogeneity of grown α-NiSO4×6H2O crystal. The crystal structures of α-NiSO4×6H2O was refinement by Rietveld method. The calculated lattice parameters are: a = 6.8000 Å, c = 18.3161 Å, Z=4, space group P41212. Four plane-parallel plates with different orientation were prepared. The orientation of the obtained plane-parallel plates was determined by XRD and are as follows: (301), (001), (320) and (204). The optical characteristics – transmittance and optical energy gap of the crystal plates were determined. All the crystal plates are characterized by higher transmittance values in the UVB range than in the VIS and NIR. The transmittance of single crystal plates decreases in the order (204) > (301) > (320) > (001). The band gap Eg values were determined by Tauc equation. Obtained Eg are in the range of 6.43–6.47 eV.
References
Maneva, M., Rizova, D., Genov, L., Liptay, G. (1999). On the thermal decomposition of NiSO4·nH2O (n=7,6,4,1) and of their deuterated analogs. Journal of Thermal Analysis. 36, 915–922. https://doi.org/10.1007/BF01904627
Fortes, A.D., Knight, K.S., Gibbs, A.S., Wood, I.G. (2018). Crystal structures of NiSO4·9H2O and NiSO4·8H2O: magnetic properties, stability with respect to morenosite (NiSO4·7H2O), the solid-solution series (MgxNi1−x)SO4·9H2O. Phys Chem Minerals. 45, 695–712. https://doi.org/10.1007/s00269-018-0956-z
Sejkora, J., Škácha, P., Vrtiška, L., Dolníček, Z., Jindra, J. (2023). Retgersite from mine dump of the Lill shaft, Březové Hory ore district, Příbram (Czech Republic). Bulletin Mineralogie Petrologie. 31(1), 89–94. https://doi.org/10.46861/bmp.31.089
Arbeck, D., Haussühl, E., Bayarjagal, L., Winkler, B., Paulsen, N., Haussühl, S., Milman, V. (2010). Piezoelastic properties of retgersite determined by ultrasonic measurements. Eur. Phys. J. B73, 167–175. https://doi.org/10.1140/epjb/e2009-00423-9
Aswathappa, S., Dai, L., Sathiyadhas, S.J.D., Amalapushpam, M.B.D.S., Thangavel, V., Vijayakumar, V.N., Kumar, R.S., Almansour, A.I. (2024). Unveiling the correlation between the structure and property of the amorphous state of hydrated nickel sulfate (NiSO4·6H2O) induced by acoustic shock waves – An X-ray diffraction, thermal calorimetric and dielectric spectroscopic approach. Materials Science and Engineering: B. 302, 117205. https://doi.org/10.1016/j.mseb.2024.117205.
Matsumoto, A., Ozawa, H., Inumarua, A., Soai, K. (2015). Asymmetric induction by retgersite, nickel sulfate hexahydrate, in conjunction with asymmetric autocatalysis. New J. Chem. 39. 6742–6745. https://doi.org/10.1039/C5NJ01459J .
Guezane Lakoud, S., Merabet-Khelassi, M., Aribi-Zouioueche, L. (2016). NiSO4·6H2O as a new, efficient, and reusable catalyst for the α-aminophosphonates synthesis under mild and eco-friendly conditions. Res Chem Intermed. 42, 4403–4415. https://doi.org/10.1007/s11164-015-2283-z.
Li, L., Chavan S., Ganjkhanlou Y., Groppo E., Sagstuen E., Bordiga S., Olsbye U., Jens K.-J. (2022). Characterization of the NiSO4 site on a NiSO4-ReOx/γ-Al2O3 catalyst for tandem conversion of ethylene to propylene. Applied Catalysis A: General. 637, 118598. https://doi.org/10.1016/j.apcata.2022.118598.
Ling Y., Chen X., Tong H., Guan W., Chen P., Huang Z., Liang C. (2021). Modulating the Interaction of NiSO4 and Nb2O5 Boosts the Dimerization of Propylene. Industrial & Engineering Chemistry Research. 60 (19), 6959-6970. https://doi.org/10.1021/acs.iecr.1c00142.
Shoair A.G.F., Almalki A.S.A., Shanab M.M.A.H., Sheta A.M., El-Basiony A., El-Ghamaz N.A., Nasef H.A., Khalaf H.A. (2023) Unlocking the Potential of NiSO4·6H2O/NaOCl/NaOH Catalytic System: Insights into Nickel Peroxide as an Intermediate for Benzonitrile Synthesis in Water. J. Nanotechnol. 2023, 9940845. https://doi.org/10.1155/2023/9940845 .
Pan Y., Chen D., Fan Y., Zuo J., Yang Q., Qiu F., Qiu L., Song H., Zhang S. (2023). Highly-sensitive and anti-interferential electrochemical determination of hazardous metronidazole using w-NiSO4·NiS2 coated ZIF-67-derived cobalt/nitrogen-doped carbon. Colloids Surf., A. 666, 131293. https://doi.org/10.1016/j.colsurfa.2023.131293.
Ali A.-R., Lackner J., Cerdas F., Herrmann C. (2023). Analysis of nickel sulphate datasets used in lithium-ion batteries. Procedia CIRP. 116, 348-353. https://doi.org/10.1016/j.procir.2023.02.059.
Ma, Y., Svärd, M., Xiao X., Sahadevan, S.A., Gardner, J., Olsson, R.T., Forsberg, K. (2022). Eutectic freeze crystallization for recovery of NiSO4 and CoSO4 hydrates from sulfate solutions. Sep. Purif. Technol. 286, 120308. https://doi.org/10.1016/j.seppur.2021.120308.
Li W., Qu J.-e., Cao Z., Wang, H. (2020) Effects of NiSO4 Concentration on the Coloring Performance and Corrosion Resistance of the Colored Film on 304 Stainless Steel. Coatings. 10, 598. https://doi.org/10.3390/coatings10060598
Thirupathy J. (2021). An Investigation of the Thermal and Dielectric Properties of Nickel Sulfate Hexahydrate Single Crystal for Sensors, Bandpass Filters and Optical Applications. J. Electron. Mater. 50, 3385–3391. https://doi.org/10.1007/s11664-021-08830-x .
Kathiravan, P., Balakrishnan, T., Srinath, C., Ramamurthi, K., Thamotharan S. (2016). Growth and characterization of α-nickel sulphate hexahydrate single crystal. Karbala International Journal of Modern Science. 2, 226–238. https://doi.org/10.1016/j.kijoms.2016.08.002 .
Masilamani, V., Shanthi, J., Sheelarani, V. (2014). Growth and Analysis of NSH and KMNSH Crystals by Slow Evaporation Technique. International Scholarly Research Notices. 678567. https://doi.org/10.1155/2014/678567.
George R., Patel I.B., Rathod K.T. (2021) Growth and photoluminescence study of nickel sulfate doped Zinc tris-Thiourea Sulfate (ZTS) crystal. Mater. Today: Proc. 37 (2), 2189–2192. https://doi.org/10.1016/j.matpr.2020.07.649 .
Eksmaoptics, Optical Filters https://eksmaoptics.com/optical-components/optical-filters/
Key Photonics, Optical Filters https://www.key-photonics.co.uk/optical-filters.php
Oliveira Neto J.G. de, Marques J.V., Silva Filho J.G. da, Antonelli E., Ayala A.P., Santos A.O. dos, Lang R. (2024) Mixed (NH4)2Mn0.47Cu0.53(SO4)2(H2O)6 Tutton salt: A novel optical material for solar-blind technology. Optical Materials. 157(3), 116400. https://doi.org/10.1016/j.optmat.2024.116400.
Kalra A., Muazzam U.U.l., Muralidharan R., Raghavan S., Nath D.N. (2022). The road ahead for ultrawide bandgap solar-blind UV photodetectors. J. Appl. Phys. 131(15), 150901. https://doi.org/10.1063/5.0082348
Hemmati, M., Rezagholipour, D. H. (2012). Unidirectional growth of α-NiSO4·6H2O crystal by Sankaranarayanan–Ramasamy (SR) method. Crystal Research and Technology. 47, 703–706. https://doi.org/10.1002/crat.201200011.
Su G., He Y., Li Z., Jiang R., Zhu C., Yang S. (2000). Directional solution growth of cylindrical α-NiSO4·6H2O crystal. Journal of Crystal Growth. 213, 99-102. https://doi.org/10.1016/S0022-0248(00)00304-3.
Anthony J.W., Bideaux R.A., Bladh K.W., Nichols M.C. (2003). Handbook of mineralogy, Vol. V. Borates, Carbonates, Sulfates. Mineral Data Publishing, Tucson, Arizona.
Jenssen I.B., Bøckman O., Andreassen J.-P., Ucar S. (2021). The Effect of Reaction Conditions and Presence of Magnesium on the Crystallization of Nickel Sulfate. Crystals. 11, 1485. https://doi.org/10.3390/cryst11121485.
Sivakumar A,. Sahaya Jude Dhas S., Thirupathy J., Reddy K.P.J., Kumar R.S., Almansour A.I., Chakraborty S., Martin Britto Dhas S.A. (2022). Switchable crystal–amorphous states of NiSO4·6H2O induced by a Reddy tube. New Journal of Chemistry. 46, 5091–5099. http://doi.org/10.1039/D2NJ00129B.
Gerkin R.E., Reppart W.J. (1988). Structure of monoclinic nickel(II) sulfate hexahydrate. Acta Crystallographica Section C. 44, 1486-1488. https://doi.org/10.1107/S0108270188004238.
Filep M., Molnár K., Sabov M., Csoma Z., Pogodin A. (2021). Structural, thermal, and optical properties of Co2+ and Mg2+ doped K2Ni(SO4)2•6H2O single crystals. Optical Materials. 122, Part A. 111753. https://doi.org/10.1016/j.optmat.2021.11175 3.
Altomare A., Cuocci C., Giacovazzo C., Moliterni A., Rizzi R., Corriero N., Falcicchio A. (2013). A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 46, 1231–1235. https://doi.org/10.1107/S0021889813013113
Xiao J.; Song Y.; Li Y. (2023). Comparison of Quantitative X-ray Diffraction Mineral Analysis Methods. Minerals. 13, 566. https://doi.org/10.3390/min13040566.
Runčevski T., Brown C.M. (2021) The Rietveld Refinement Method: Half of a Century Anniversary. Cryst. Growth Des. 21(9), 4821-4822. https://doi.org/10.1021/acs.cgd.1c00854 .
Momma K., Izumi F. (2011). VESTA 3 for three-dimen-sional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276. https://doi.org/10.1107/S0021889811038970
Triest M., Bussière G., Bélisle H., Reber C. (2000). Why does the middle band in the absorption spectrum of Ni(H2O)62+ have two maxima? J. Chem. Educ. 77, 670. https://doi.org/10.1021/ed077p670.2.
Allaham M., Dallaev R., Burda D., Sobola D., Nebojsa A., Knápek A., Mousa M.S., Kolařík V. (2024). Energy gap measurements based on enhanced absorption coefficient calculation from transmittance and reflectance raw data. Phys. Scr. 99 (2), 025952. https://doi.org/10.1088/1402-4896/ad1cb8.
Jubu, P.R., Danladi, E., Ndeze, U.I., Adedokun, O., Landi, S., Haider, A.J., Adepoju, A.T., Yusof, Y., Obaseki, O.S., Yam F.K. (2024) Comment about the use of unconventional Tauc plots for bandgap energy determination of semiconductors using UV–Vis spectroscopy. Results in Optics. 14, 100606. https://doi.org/10.1016/j.rio.2024.100606.
Haryński, Ł., Olejnik, A., Grochowska, K., Siuzdak, K. (2022). A facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV–Vis spectroscopy data. Optical Materials. 127, 112205. https://doi.org/10.1016/j.optmat.2022.112205
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

