HALOGENOCYCLIZATION OF TERMINAL 2-BUTYNYL(PENTYNYL)THIOQUINOLINE-3-CARBALDEHYDE
DOI:
https://doi.org/10.15421/jchemtech.v32i4.316454Keywords:
2-(but-1-yn-4-ylthio)quinoline-3-carbaldehyde, 2-(pent-1-yn-5-ylthio)quinoline-3-carbaldehyde, haloheterocyclization, 1-halomethylidene-4-formyl-1,2-dihydro[1,3]-thiazino[3,2-a]quinolinium, 1-halomethylidene-6-formyl-1,2,3,4-tetrahydro[1,3]thiazepino[3,2-a]quinolinium, monohalide, trihalideAbstract
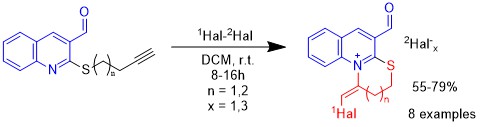
Polycondensed functional quinoline derivatives exhibit various biological activities. Annulating of nitrogen-containing heterocycles to 3-functionalized quinoline expands the possibilities for discovering bioactive compounds. The electrophilic cyclization of alkylunsaturated thioderivatives of quinoline, under the action of halogen-containing electrophiles, creates prerequisites for the synthesis of polycondensed heterocycles based on quinoline. This study investigates the regio- and stereochemistry of the process of electrophilic intramolecular cyclization of terminal 2-butynyl(pentynyl)thioquinoline-3-carbaldehyde under the influence of molecular and hybrid halogens. For the first time, long-chain alkynyl thioethers of quinoline-3-carbaldehyde were synthesized, which effectively underwent electrophilic heterocyclization reactions under the action of molecular and hybrid halogens. The type of ring fused to quinoline depends on the length of the alkynyl substituent and is independent of the nature of the halogenating agent. It was determined that when bromine acts on alkynyl thioethers of quinoline, bromine-induced heterocyclization occurs, and when iodine, iodine bromide, or iodine chloride act, iodine-induced halogenoheterocyclization occurs. The cyclization process is stereoselective, forming 1-halomethylidene-4-formyl-1,2-dihydro[1,3]thiazino[3,2-a]quinolinium and 1-halomethylidene-6-formyl-1,2,3,4-tetrahydro[1,3]thiazepino-[3,2-a]quinolinium mono- and trihalides with E-configuration.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

