ALKYLTHIOBENZOTHIAZOLES DECORATED WITH 1,2,3-TRIAZOLE
DOI:
https://doi.org/10.15421/jchemtech.v32i4.316457Keywords:
2-(but-1-yn-4-ylthio)benzothiazole, 2-(pent-1-yn-5-ylthio)benzothiazole, cycloaddition, click-reaction, 1,2,3-triazole, ; hybrid heterocycleAbstract
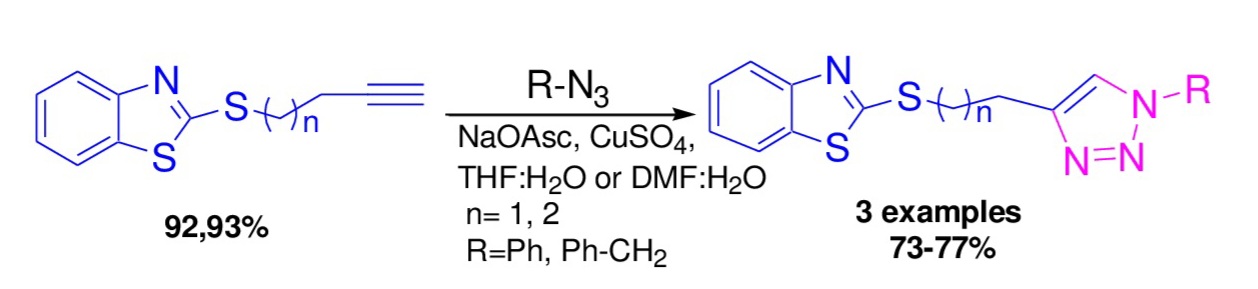
Triazole and its hybrid derivatives exhibit a range of biological activities, that is why the synthesis of hybrid heterocycles that contain triazole and benzothiazole rings is relevant. The aim of the current study is the investigation of the use of terminal butynyl and pentynyl thioethers of benzothiazole in the copper catalytic reaction of 1,3-dipolar cycloaddition of azides. 2-(but-1-yn-4-ylthio)- and 2-(pent-1-yn-5-ylthio)-benzothiazoles were synthesized for the first time in high yields via alkylation of 2-mercaptobenzothiazole with terminal butynyl and pentynyl bromides. Long-chain alkynyl thioethers of benzothiazole were used in copper catalytic reactions of 1,3-dipolar cycloaddition with aromatic and aliphatic azides. Based on experimental data, it was established that when using butynyl thioether in the reaction with phenylazide, the most effective solvent was a mixture of tetrahydrofuran and water. While the cycloaddition of pentynyl thioether with phenyl and benzyl azides was carried out in a dimethylformamide-water mixture. As a result, the new hybrid 1,2,3-triazolylthiobenzothiazoles, which can potentially exhibit high bioactivity, were synthesized in high yields and separated by two or three methylene spacers.
References
Salma, U., Ahmad, S., Alam, M. Z., Khan, S. A. (2024). Synthetic approaches and biological applications of triazole derivatives. Mol. Struct., 1301(137240). https://doi.org/10.1016/j.molstruc.2023.137240
Fan, Y. L., Ke, X., Liu, M. (2018). Coumarin–triazole hybrids and their biological activities. J. Heterocycl. Chem., 55(4), 791–802. https://doi.org/10.1002/jhet.3112
Phatak, P. S., Sathe, B. P., Dhumal, S. T., Rehman, N. N., Dixit, P. P., Khedkar, V. M., Haval, K. P. (2019). Synthesis, Antimicrobial Evaluation, and Docking Studies of Substituted Acetylphenoxymethyl‐triazolyl‐N‐phenylacetamides. J. Heterocycl. Chem., 56(7), 1928–1938. https://doi.org/10.1002/jhet.3568
Jadhav, R. P., Raundal, H. N., Patil, A. A., Bobade, V. D. (2017). Synthesis and biological evaluation of a series of 1, 4-disubstituted 1, 2, 3-triazole derivatives as possible antimicrobial agents. J. Saudi Chem. Soc., 21(2), 152–159. https://doi.org/10.1016/j.jscs.2015.03.003
Yadav, A., Kaushik, C. P. (2022). Synthesis and antibacterial evaluation of sulfonamide bridged disubstituted 1, 2, 3-triazoles. Synth. Commun., 52(24), 2261–2275. https://doi.org/10.1080/00397911.2022.2141126
Bo Zhang (2019). Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem., 168, 357–372. https://doi.org/10.1016/j.ejmech.2019.02.055
Marinescu, M. (2023). Benzimidazole-triazole hybrids as antimicrobial and antiviral agents: A systematic review. Antibiotics, 12(7), 1220. https://doi.org/10.3390/antibiotics12071220
Dwivedi, B., Bhardwaj, D., Choudhary, D. (2024). Green design and synthesis of some novel thiazolidinone appended benzothiazole–triazole hybrids as antimicrobial agents. RSC advances, 14(12), 8341–8352. https://doi.org/10.1039/D4RA00990H
Marzi, M., Farjam, M., Kazeminejad, Z., Shiroudi, A., Kouhpayeh, A., Zarenezhad, E. (2022). A recent overview of 1, 2, 3‐triazole‐containing hybrids as novel antifungal agents: Focusing on synthesis, mechanism of action, and structure‐activity relationship (SAR). Journal of Chemistry, 2022(1), 7884316. https://doi.org/10.1155/2022/7884316
Subhashini, N. J. P., Kumar, E. P., Gurrapu, N., Yerragunta, V. (2019). Design and synthesis of imidazolo-1, 2, 3-triazoles hybrid compounds by microwave-assisted method: Evaluation as an antioxidant and antimicrobial agents and molecular docking studies. J. Mol. Struct., 1180, 618–628. https://doi.org/10.1016/j.molstruc.2018.11.029
Chavan, P. V., Desai, U. V., Wadgaonkar, P. P., Tapase, S. R., Kodam, K. M., Choudhari, A., Sarkar, D. (2019). Click chemistry based multicomponent approach in the synthesis of spirochromenocarbazole tethered 1, 2, 3-triazoles as potential anticancer agents. Bioorg. Chem., 85, 475–486. https://doi.org/10.1016/j.bioorg.2019.01.070
Çeşme, M., Onur, S., Aksakal, E. Tümer, F. (2024). Novel hybrid structures based on 4-Chlorobenzenesulfonyl and 1,2,3-triazoles: Synthesis, in vitro biological activities and in silico studies. J. Mol. Liq., 409, 125501. https://doi.org/10.1016/j.molliq.2024.125501
El Azab, I.H., El-Sheshtawy, H.S., Bakr, R.B., Elkanzi N.A.A. (2021). New 1,2,3-Triazole-Containing Hybrids as Antitumor Candidates: Design, Click Reaction Synthesis, DFT Calculations, and Molecular Docking Study. Molecules, 26(3), 708. https://doi.org/10.3390/molecules26030708
Menendez, C., Gau, S., Lherbet, C., Rodriguez, F., Inard, C., Pasca, M. R., Baltas, M. (2011). Synthesis and biological activities of triazole derivatives as inhibitors of InhA and antituberculosis agents. Eur. J. Med. Chem., 46(11), 5524–5531. https://doi.org/10.1016/j.ejmech.2011.09.013
Zhang, S., Xu, Z., Gao, C., Ren, Q. C., Chang, L., Lv, Z. S., Feng, L. S. (2017). Triazole derivatives and their anti-tubercular activity. Eur. J. Med. Chem., 138, 501–513. https://doi.org/10.1016/j.ejmech.2017.06.051
Haleha, O. V., Povidaichyk, M. V., Svalyavin, O. V., Ostapchuk, E. M., Onysko, M. Y. (2023). [Synthesis and conversion of thiazinobenzothiazolium salts]. Voprosy khimii i khimicheskoi tekhnologii., 2, 61–66. (in Ukrainian). http://dx.doi.org/10.32434/0321-4095-2023-147-2-61-66
Haleha, O. V., Povidaichyk, M. V., Komarovska-Porokhnyavets, O. Z., Onysko, M. Y., Sukharev S. М. [Synthesis and antimicrobial activity of seleno(mercury)halogen-containing benzothiazole derivatives]. Sci. Bull. Uzhh. Univ. Ser. Chem., 49(1), 39–44. (in Ukrainian). https://doi.org/10.24144/2414-0260.2023.1.39-44
Shafi, S., Alam, M. M., Mulakayala, N., Mulakayala, C., Vanaja, G., Kalle, A. M., Alam, M. S. (2012). Synthesis of novel 2-mercapto benzothiazole and 1, 2, 3-triazole based bis-heterocycles: their anti-inflammatory and anti-nociceptive activities. Eur. J. Med. Chem., 49, 324–333. https://doi.org/10.1016/j.ejmech.2012.01.032
Mir, F., Shafi, S., Zaman, M. S., Kalia, N. P., Rajput, V. S., Mulakayala, C., Alam, M. S. (2014). Sulfur rich 2-mercaptobenzothiazole and 1, 2, 3-triazole conjugates as novel antitubercular agents. Eur. J. Med. Chem., 76, 274–283. https://doi.org/10.1016/j.ejmech.2014.02.017
Kuribayashi, S., Shida, N., Inagi, S., Fuchigami, T. (2016). Synthesis of fluorinated triazole and isoxazole derivatives by electrochemical fluorination. Tetrahedron, 72(35), 5343–5349. https://doi.org/10.1016/j.tet.2016.07.016
Gong, Z., Peng, Y., Qiu, J., Cao, A., Wang, G., Peng, Z. (2017). Synthesis, In Vitro α-Inhibitory Activity and Molecular Docking Studies of Novel Benzothiazole-Triazole Derivatives. Molecules, 22(9), 1555. https://doi.org/10.3390/molecules22091555
Fizer, M., Slivka, M.; Baumer, V.; Slivka, M.; Fizer, O. (2019). Alkylation of 2-oxo(thioxo)-thieno[2,3-d]pyrimidine-4-ones: Experimental and theoretical study. Journal of Molecular Structure, 1198, 126858. https://doi.org/10.1016/j.molstruc.2019.07.105
Jaiswal, S., Devi, M., Sharma, N., Rathi, K., Dwivedi, J., Sharma, S. (2022). Emerging approaches for synthesis of 1, 2, 3-triazole derivatives. a review. Organic Preparations and Procedures International, 54(5), 387–422. https://doi.org/10.1080/00304948.2022.2069456
Reddy, G. S., Reddy, L. M., Kumar, A. S., Ramachary, D. B. (2020). Organocatalytic Selective [3+ 2] Cycloadditions: Synthesis of Functionalized 5-Arylthiomethyl-1, 2, 3-triazoles and 4-Arylthio-1, 2, 3-triazoles. The Journal of Organic Chemistry, 85(23), 15488–15501. https://doi.org/10.1021/acs.joc.0c02247
Kumar, S., Lal, B., Tittal, R. K. (2024). Green Synthesis of 1, 2, 3-Triazoles: A Sustainable Approach. Green Chemistry. https://doi.org/10.1039/D3GC04346
Hu, H., Ohno, A., Sato, T., Mase, T., Uozumi, Y., Yamada, Y. M. (2019). Self-assembled polymeric pyridine copper catalysts for Huisgen cycloaddition with alkynes and acetylene gas: application in synthesis of tazobactam. Org. Process Res. Dev., 23(4), 493–498. https://doi.org/10.1021/acs.oprd.8b00429
Trujillo, M., Hull-Crew, C., Outlaw, A., Stewart, K., Taylor, L., George, L., .Schoffstall, A. (2019). Green methodologies for copper (I)-catalyzed azide-alkyne cycloadditions: a comparative study. Molecules, 24(5), 973. https://doi.org/10.3390/molecules24050973
Rzonsowska, M., Kozakiewicz, K., Mituła, K., Duszczak, J., Kubicki, M., Dudziec, B. (2021). Synthesis of silsesquioxanes with substituted triazole ring functionalities and their coordination ability. Molecules, 26(2), 439. https://doi.org/10.3390/molecules26020439
Mittersteiner, M., Aquino, E. C., Budragchaa, T., Wessjohann, L. A., Bonacorso, H. G., Martins, M. A., Zanatta, N. (2022). Synthesis of Methylene-Bridged Trifluoromethyl Azoles Using 5-(1, 2, 3-Triazol-1-yl) enones. Synthesis, 54(02), 439–450. https://doi.org/10.1055/s-0040-1719837
Bagra, N., Jain, R. (2022). Synthesis of 4-(1, 2, 3-triazol-1-yl)-L-phenylalanines. Synthetic Communications, 52(8), 1176–1183. https://doi.org/10.1080/00397911.2022.2077114
Nural, Y., Ozdemir, S., Doluca, O., Demir, B., Yalcin, M. S., Atabey, H., Seferoglu, Z. (2020). Synthesis, biological properties, and acid dissociation constant of novel naphthoquinone–triazole hybrids. Bioorganic Chemistry, 105, 104441. https://doi.org/10.1016/j.bioorg.2020.104441
Igual, M. O., Nunes, P. S., da Costa, R. M., Mantoani, S. P., Tostes, R. C., Carvalho, I. (2019). Novel glucopyranoside C2-derived 1, 2, 3-triazoles displaying selective inhibition of O-GlcNAcase (OGA). Carbohydrate research, 471, 43–55. https://doi.org/10.1016/j.carres.2018.10.007
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

