SPECTROPHOTOMETRIC DETERMINATION OF THIAMINE USING OXIDATION REACTION WITH 18-MOLYBDODIPHOSPHATE
DOI:
https://doi.org/10.15421/jchemtech.v33i1.321291Keywords:
spectrophotometry, thiamine, 18-molybdodiphosphate, pharmaceuticalsAbstract
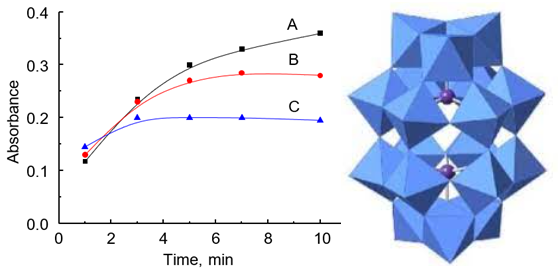
A simple and highly sensitive method for the determination of thiamine in pharmaceuticals has been developed. It is based on the reaction between thiamine and 18-molybdodiphosphate heteropoly complex (18-MPC), which occurs at pH≈10, created by the addition of 10 % sodium carbonate. The reaction takes place at room temperature. 7-minutes are sufficient for the complete reduction of 18-MPC. The stability of heteropolyblue is limited and absorbance significantly decreases after 10 minutes of reaction. The optimal concentrations of 18-MPC and sodium carbonate were found to be 0.64 mM and 2.4 %, respectively. The ratio of 18-MPC to thiamine in the reaction is 2 : 1, which is shown by the method of continuous variations. Four electrons are accepted by the thiamine molecule during the oxidation of 18-MPC. In this case, a two-electron heteropolyblue is formed with an absorption maximum at 820 nm. The calibration dependence is linear in the range of thiamine concentrations from 2 to 80 μM with a detection limit of 0.8 μM using a cuvette with 0.5 cm path length. The proposed method has been successfully applied to the determination of vitamin B1 in three different pharmaceutical formulations containing other B vitamins, vitamin B6 and vitamin B12.
References
Mrowicka, M., Mrowicki, J., Dragan, G., Majsterek, I. (2023). The importance of thiamine (vitamin B1) in humans. Bioscience Reports., 43(10), BSR20230374. https://doi.org/10.1042/BSR20230374
Cizmarova, I., Matuskova, M., Stefanik, O., Horniakova, A., Mikus, P., Piestansky, J. (2022). Determination of thiamine and pyridoxine in food supplements by a green ultrasensitive two-dimensional capillary electrophoresis hyphenated with mass spectrometry. Chem. Pap., 76(10), 6235–6245. https://doi.org/10.1007/s11696-022-02309-7
Verstraete, J., Stove, C. (2021). Patient-centric assessment of thiamine status in dried blood volumetric absorptive microsamples using LC–MS/MS analysis. Anal. Chem., 93(4), 2660–2668. https://doi.org/10.1021/acs.analchem.0c05018
Mathew, E. M., Sakore, P., Lewis, L., Manokaran, K., Rao, P., Moorkoth, S. (2019). Development and validation of a dried blood spot test for thiamine deficiency among infants by HPLC – fluorimetry. Biomed. Chromatogr., 33(11), e4668. https://doi.org/10.1002/bmc.4668
Rocchi, R., van Kekem, K., Heijnis, W. H., Smid, E. J. (2022). A simple, sensitive, and specific method for the extraction and determination of thiamine and thiamine phosphate esters in fresh yeast biomass. J. Microbiol. Methods, 201, 106561. https://doi.org/10.1016/j.mimet.2022.106561
Zhang, M., Zhang, C., Cai, H., Xie, Y., Sun, M., Liu, J., Liu, J., Yi, C., Fan, H., Yi, W., Lv, Z. (2025). Rapid determination of water-soluble vitamins in human serum by ultrahigh-performance liquid chromatography-tandem mass spectrometry. ACS Omega, 10, 885–891. https://doi.org/10.1021/acsomega.4c07968
Momeni, S., Jaberie, H. (2025). Developing a highly efficient fluorescence strategy for thiamine detection in real samples. J. Photochem. Photobiol. A, 459, 116064. https://doi.org/10.1016/j.jphotochem.2024.116064
Rocha, F. R. P., Filho, O. F., Reis, B. F. (2003). A multicommuted flow system for sequential spectrophotometric determination of hydrosoluble vitamins in pharmaceutical preparations. Talanta, 59, 191–200. https://doi.org/10.1016/S0039-9140(02)00477-0
Al-Ahmary, K. M. (2014). A simple spectrophotometric method for determination of thiamine (vitamin B1) in pharmaceuticals. Eur. J. Chem., 5(1), 81–84. https://doi.org/10.5155/eurjchem.5.1.81-84.881
Abo Dena, A. S., Ammar, A. A. (2019). H-point standard addition for simultaneous reagent-free spectrophotometric determination of B1 and B6 vitamins H-point standard addition for simultaneous reagent-free spectrophotometric determination of B1 and B6 vitamins. Spectrochim Acta A, 206, 491–497. https://doi.org/10.1016/j.saa.2018.08.047
Al Abachi, M. Q., Hadi, H. (2012). Normal and reverse flow injection–spectrophotometric determination of thiamine hydrochloride in pharmaceutical preparations using diazotized metoclopramide. J. Pharm. Anal., 2(5), 350–355. https://doi.org/10.1016/j.jpha.2012.04.005
Liu, S., Zhang, Z., Liu, Q., Luo, H., Zheng, W. (2002). Spectrophotometric determination of vitamin B 1 in a pharmaceutical formulation using triphenylmethane acid dyes. J. Pharm. Biomwd. Anal. 30, 685–694. https://doi.org/10.1016/S0731-7085(02)00356-4
Vishnikin, A. B., Miekh, Y. V., Denisenko, T. A., Kozhemiaka, V. G., Vishnikina, V. Yu., Al-Shwaiyat, M. K. E. A., Bazel, Ya. R., Andruch, V. (2018). Determination of thiamine as a complex with 11-molybdobismutho(III)phosphate in sequential injection Lab-at-valve system. Methods Objects Chem. Anal., 13, 51–59. https://doi.org/10.17721/moca.2018.55-63
Tsiganok, L. P., Vishnikin, A. B., Maksimovskaya, R. I. (1989). UV, IR, 71Ga and 17O NMR spectroscopic studies of 12-molybdogallate. Polyhedron, 23, 2739–2742. https://doi.org/10.1016/S0277-5387(00)80529-X
Vishnikin, A. B., Al-Shwaiyat, M. K. E. A., Bazel, Y. R., Andruch, V. (2007). Rapid, sensitive and selective spectrophotometric determination of phosphate as an ion associate of 12-molybdophosphate with Astra Phloxine. Microchim. Acta, 159, 371–378. https://doi.org/10.1007/s00604-007-0754-7
Biocic, M., Kraljevic, T., Spassov, T. G., Kukoc-Modun, L., Kolev, S. D. (2024). Sequential injection analysis method for the determination of glutathione in pharmaceuticals. Sensors, 24(17), 5677. https://doi.org/10.3390/s24175677
Mogashane, T. M., Mapazi, O., Motlatle, M. A., Mokoena, L., Tshilongo, J. (2025). A review of recent developments in analytical methods for determination of phosphorus from environmental samples. Molecules, 30(5), 1001. https://doi.org/10.3390/molecules30051001
Perez, M., Dominguez-Lopez, I., Lamuela-Raventos, R. (2023). The chemistry behind the Folin–Ciocalteu method for the estimation of (poly)phenol content in food: total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem., 71, 17543–17553. https://doi.org/10.1021/acs.jafc.3c04022
Al-Shwaiyat, M., Denisenko, T., Miekh, Y., Vishnikin, A. (2018). Spectrophotometric determination of polyphenols in green teas with 18-molybdodiphosphate. Chem. Chem. Technol., 12(2), 135–142. https://doi.org/10.23939/chcht12.02.135
Vishnikin, A., Miekh, Yu., Denisenko, T., Bazel, Ya., Andruch, V. (2018). Use of sequential injection analysis with lab-at-valve and optical probe for simultaneous spectrophotometric determination of ascorbic acid and cysteine by mean centering of ratio kinetic profiles. Talanta, 188, 99–106. https://doi.org/10.1016/j.talanta.2018.05.056
Vishnikin, A. B., Sklenařova, H., Solich, P., Petrushina, G. A., Tsiganok, L. P. (2011). Determination of ascorbic acid with Wells-Dawson type molybdophosphate in sequential injection system. Anal. Lett., 44(1-3), 514–527. https://doi.org/10.1080/00032719.2010.500789
Vishnikin, A. B., Svinarenko, T. Ye., Sklenářová, H., Solich, P., Bazel, Ya. R., Andruch, V. (2010). 11-Molybdobismuthophosphate - a new reagent for the determination of ascorbic acid in batch and sequential injection systems. Talanta, 80, 1838–1845. https://doi.org/10.1016/j.talanta.2009.10.031
Vishnikin, A. B., Al-Shwaiyat, M. K. E. A., Petrushina, G. A., Tsiganok, L. P., Andruch, V., Bazel, Ya. R., Sklenarova, H., Solich, P. (2012). Highly sensitive sequential injection determination of p-aminophenol in paracetamol formulations with 18-molybdodiphosphate heteropoly anion based on elimination of Schlieren effect. Talanta, 96, 230–235. https://doi.org/10.1016/j.talanta.2012.02.049
Petrova, A., Ishimatsu, R., Nakano, K., Imato, T., Vishnikin, A. B., Moskvin, L. N., Bulatov, A. V. (2016). Flow-injection spectrophotometric determination of cysteine in biologically active dietary supplements. J. Anal. Chem., 71(2), 172–178. https://doi.org/10.1134/S1061934816020118
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

