SYNTHESIS OF THIOAMIDE COMPLEX COMPOUNDS OF COPPER(II) AND STUDY OF THEIR MUTUAL TRANSFORMATIONS
DOI:
https://doi.org/10.15421/jchemtech.v33i2.321442Keywords:
thioamides; complexation reactions; heterocyclic thioamide ligands; stepwise hydrolysis; copper(II) complexes.Abstract
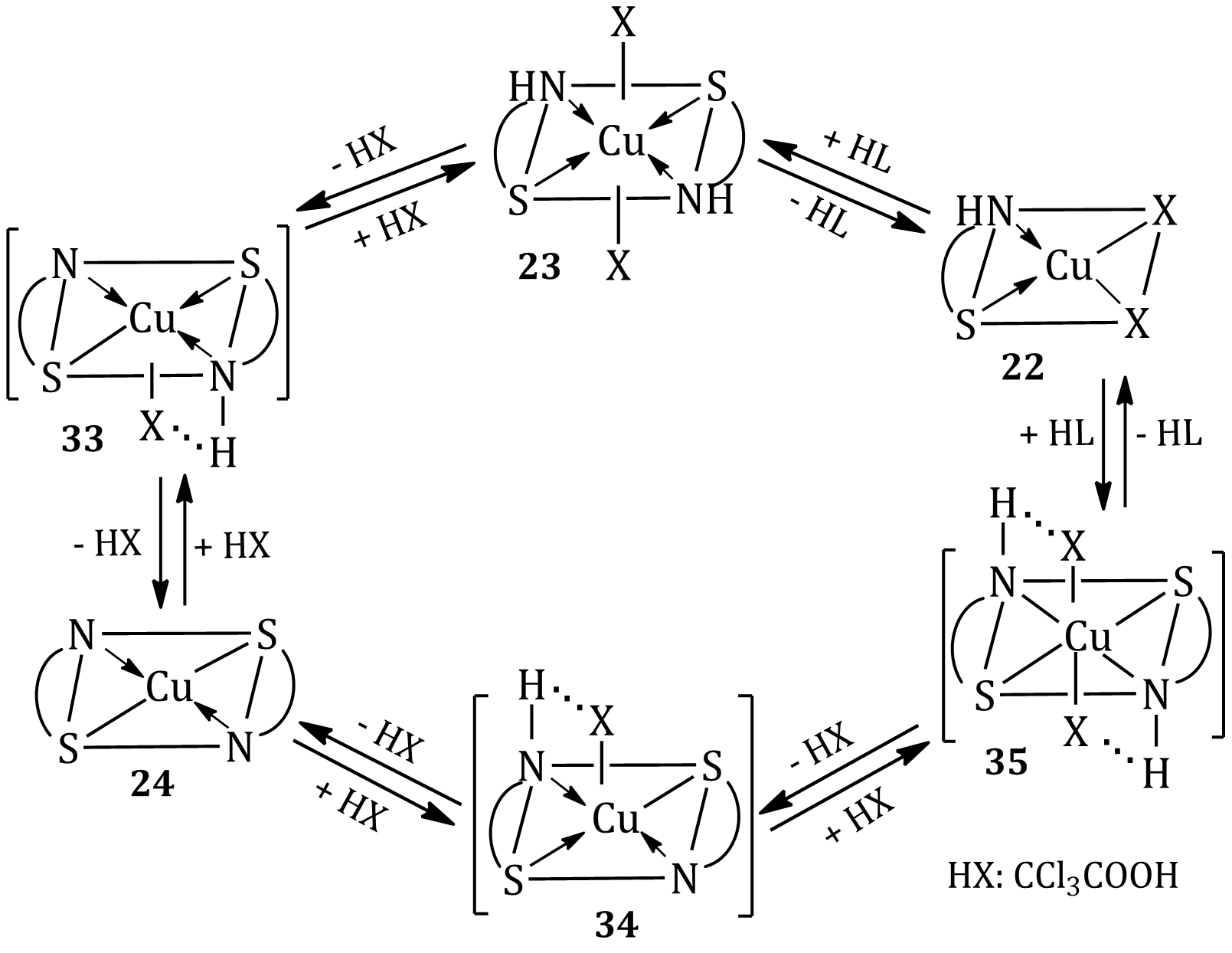
The paper presents improved methods of the synthesis of thioamides of various substitution RC(=S)NHR' (R = R' = Alk, Ar, Het), which is the promising chelating ligandsfor complexation reaction with some 3d-metals. Coordination compounds of copper(II) of the composition [Cu(НL)2X2]2, [Cu(НL)X2]2, [CuL2]2, containing neutral or deprotonated molecules of benzimidazole-2-N-(4-methoxyphenyl)carbothioamide (HL) and trichloroacetate anion (Х–) were synthesized. The dimer di(trichloroacetato)-[benzimidazole-2-N-(4-methoxyphenyl)carbothioamide] copper(II) was obtained by the interaction of thioamide (HL) with copper(II) trichloroacetate and trichloroacetic acid in an alcohol medium. The conditions for the transformation (temperature, time, solvent, pH) of one form of the coordination compound into another [Cu(НL)X2]2 ↔ [CuL2]2 ↔ [Cu(НL)2X2]2 have been determined. The study showed that the synthesis of such coordination compounds in methanol or water-methanol solutions depends on the acid-base properties of the reaction medium. The composition and structure of the synthesized compounds during the above transformations were investigated by elemental analysis and IR spectroscopy. Thus, the possibility of targeted synthesis of functionally oriented coordination compounds [Cu(HL)2X2]2, [Cu(HL)X2]2, [CuL2]2 by their mutual transformations has been established.
References
Iagodzinski, T. S. (2003). Thioamides as useful synthons in the synthesis of heterocycles. Chem. Rev. 103(1), 197–228. https://doi.org/10.1021/cr0200015.
Priebbenow, D. L., Bolm, C. (2013). Recent advances in the Willgerodt–Kindler reaction. Chem. Soc. Rev., 42, 7870–7880. https://doi.org/10.1039/C3CS60154D.
Zhang, Q., Soulere, L., Queneau, Y. (2023). Toward More Practical Methods of the Chemical Synthesis of Thioamides Using Sulfuration Agents: A Decade Update. Molecules, 28(8), 3527. https://doi.org/10.3390/molecules28083527.
Murai, T. (2019). Synthesis of Thioamides. In: Murai, T. (eds) Chemistre of Thioamides. Springer Singapore. https://doi.org/10.1007/978-981-13-7828-7_3.
Hansen, T. N., Olsen, Ch. A. (2024). Contemporary Application of Thioamides and Methods for Their Synthesis. Chem. Eur. J., 30(9), e202303770. https://doi.org/10.1002/chem.202303770.
Chojnacki, I., Monka, M., Serdiuk, I. E., Bojarski, P., Połońskia, T., Olszewska, T. (2021). Copper(I) halide cluster-based coordination polymers modulated by chiral ditopic dithiodianthranilide ligands: synthesis, crystal structure and photoluminescence. CrystEngComm, 23, 299–307. https://doi.org/10.1039/D0CE01589J.
Lobana, T. S., Sultana, R., Hundal, G. (2008). Heterocyclic thioamides of copper(I): Synthesis and crystal structures of copper complexes with 1,3-imidazoline-2-thiones in the presence of triphenyl phosphine. Polyhedron, 27(3), 1008–1016. https://doi.org/10.1016/j.poly.2007.11.036.
Long, D. L., Zeng, D. X., Xin, X. G., Huang, X. Y., Kang, B. Sh. (1996). Synthesis and Characterization of Copper(I) and Silver(I) Complexes Containing Thioamide Ligands. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 26(5), 723–733. https://doi.org/10.1080/00945719608004331.
Gordienko, O., Titov, T., Ranskiy, A., Gumenchuk, O. (2018). Synthesis, Structure and Properties of Copper(II) Chelates with Benzimidazole-2-N-Arylcarbothioamides. Chem. Chem. Technol., 12(2), 176–181. https://doi.org/10.23939/chcht12.02.176.
Ranskiy, А., Didenko, N., Gordienko, O. (2017). Synthesis of Heterocyclic Thioamides and Сopper(II) Coordination Compounds Based on Them. Chem. Chem. Technol. 11(1), 11–18. https://doi.org/10.23939/chcht11.01.011.
Ranskiy, А., Gordienko, O., Didenko, N., Titov, T., Khutko, M. (2020). Synthesis, Structure and Application of Mixed-Ligand Coordination Compounds of Copper(II) with Substituted Thioamides. Chem. Chem. Technol., 14(1), 55–61. http://doi.org/10.23939/chcht14.01.055.
Ranskiy, А. P., Panasyuk, A. G., Aliev, Z. G. (2005). Synthesis and X-ray Diffraction Analysis of {Perchlorato-bis[benzimidasole-2-N-94-metoxyphenyl)carbothiomidato]copper(III)} Hydroperchlorate. Rus. J. Coord. Chem., 31, 40–44. https://doi.org/10.1007/PL00022086.
Orysyk, S., Pekhnyo, V., Orysyk, V., Zborovskii, Y., Borovyk, P., Mykhailo, V. (2022). Fundamental Aspects of the Coordination Chemistry of Transition Metals with Functionally Substituted Thioamides (Part 1). Ukrainian Chemistry Journal, 88(2), 85–115. https://doi.org/10.33609/2708-129X.88.02.2022.85-115.
Orysyk, S., Pekhnyo, V., Orysyk, V., Zborovskii, Y., Borovyk, P., Vovk, M. (2022). Fundamental Aspects of Coordination Chemistry of Transition Metals with Functionally Substituted Thioamides (Part 2). Ukrainian Chemistry Journal, 88(3), 3–27. https://doi.org/10.33609/2708-129X.88.03.2022.3-27.
Li, J., Ren, X., Li, G., Liang, H., Zhao, Y., Wang, Z., Li, H., Yuan, B. (2020). Mixed bases mediated synthesis of thioamides in water. J. Sulfur. Chem., 41(3), 229–237. https://doi.org/10.1080/17415993.2020.1722818.
Gupta, A.; Vankar, J. K.; Jadav, J. P.; Gururaja, G. N. (2022). Water mediated direct thioamidation of aldehydes at room temperature. J. Org. Chem., 87(5), 2410–2420. https://doi.org/10.1021/acs.joc.1c02307
Tian, H., Guo, F., Chen, X. (2022). Csp3–H bond functionalization of α-bromo ketones for the synthesis of α-keto thioamides using elemental sulfur. Russ. J. Org. Chem., 58, 1260–1266. https://doi.org/10.1134/S107042802209010X.
Tayade, Y. A., Jangale, A. D., Dalal, D. S. (2018). Simple and Highly Efficient Synthesis of Thioamide Derivatives using β-Cyclodextrin as Supramolecylar Catalist in Water. ChemistrySelect, 3(31), 8895–8900. https://doi.org/10.1002/slct.201801553.
Kale, A. D., Tayade, Y. A., Mahale, S. D., Patil, R. D., Dalal, D. S. (2019). Willgerodt-Kindler reaction at room temperature: Synthesis of thioamides from aromatic aldehydes and cyclic secondary amines. Tetrahedron, 75(41), 130574. https://doi.org/10.1016/j.tet.2019.130575.
Reiss, A., Mureseanu, M. (2012). Transition metal complexes with ligand containing thioamide moiety: synthesis, characterization and antibacterial activities. J. Chil. Chem. Soc., 57(4), 1409–1414. http://dx.doi.org/10.4067/S0717-97072012000400016.
Ranskiy, А.P., Didenko, N.O., Gordienko, O.A. (2016). [Synthesis of hetaryl-2-thiocarboxylic acid alkylamides and based on them copper(II) complexes ]. Ukr. Chem. J.. 82(8), 117–125. (in Ukrainian). https://ucj.org.ua/index.php/journal/issue/view/102/8-2016.
Ranskiy, А.P., Boychenko, S.V., Gordienko, O.A., Didenko, N.O., Voloshynets, V.A. (2012). Composite lubricants based on thioamides and their complex compounds. Synthesis. Research. Use. Monograph. Vinnytsia, VNTU. 328. (in Ukrainian).
https://press.vntu.edu.ua/index.php/vntu/catalog/book/207
Didenko, N.O., Ranskiy, А.P. (2021). Direct synthesis of copper(II) coordination compounds with substituted thioamides. Monograph. Vinnytsia, VNTU. 112. (in Ukrainian). https://press.vntu.edu.ua/index.php/vntu/catalog/book/623
Ranskiy, А.P., Evseeva M.V. (2009). Synthesis, structure and complexation reactions of aromatic and heterocyclic thioamides. Monograph. Vinnytsia, VNTU. 128. (in Ukrainian). https://press.vntu.edu.ua/index.php/vntu/catalog/book/489
Jensen, K. A., Nielsen, P. H. (1966). Infrared Spectra of Thioamides and Selenoamides. Acta Chem. Scand., 20(3), 597–629. http://actachemscand.org/pdf/acta_vol_20_p0597-0629.pdf
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

