KINETICS OF GLYCEROL ACETYLATION WITH ACETIC ACID IN THE PRESENCE OF NATURAL ALUMOSILICATES MODIFIED WITH ARENESULFONIC ACID FRAGMENTS
DOI:
https://doi.org/10.15421/jchemtech.v33i2.322096Keywords:
glycerol, acetylation, alumosilicates, acetins, kinetics, catalysisAbstract
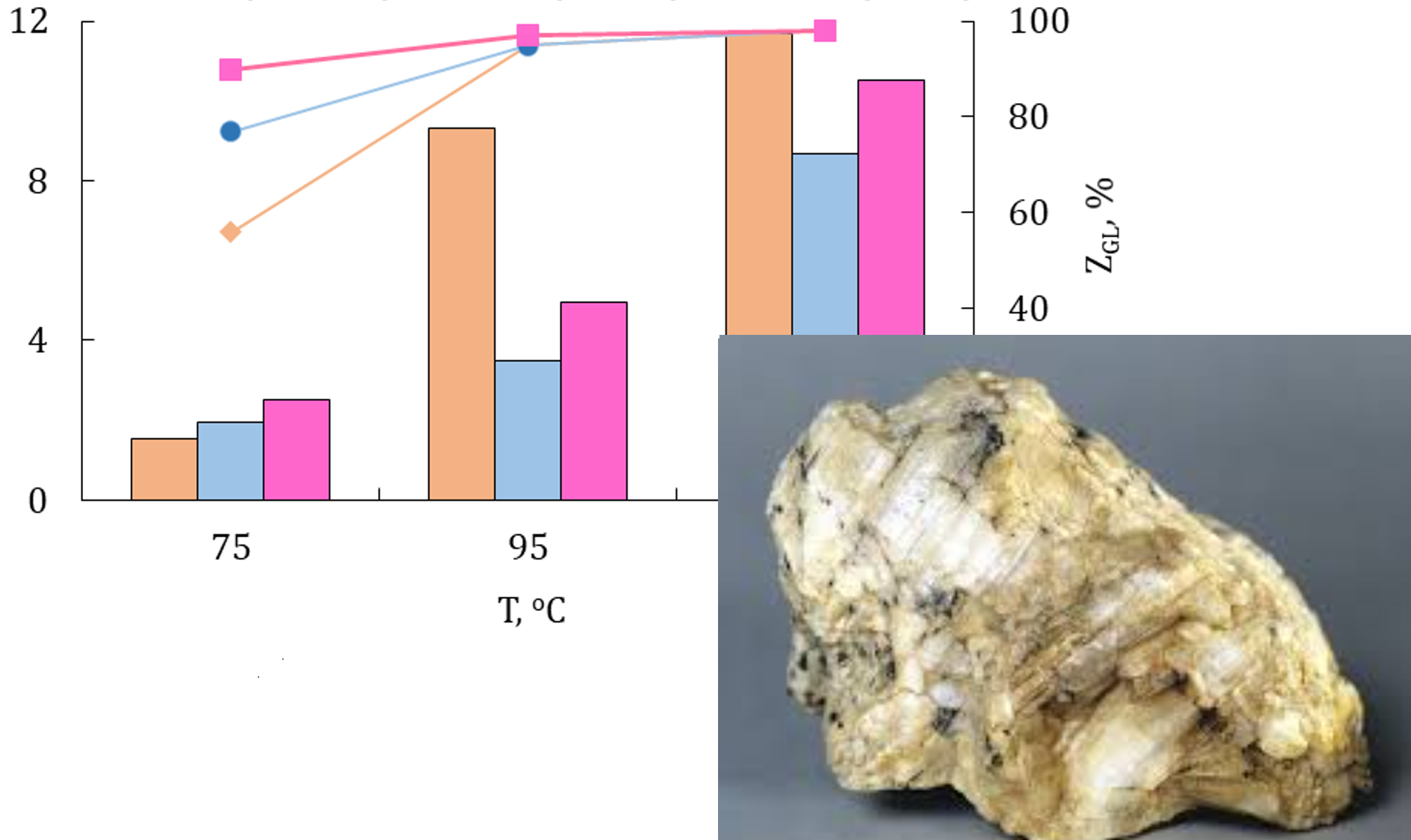
The kinetics of glycerol acetylation with acetic acid in the presence of natural aluminosilicates of the domestic deposit modified with arenesulfonic acid fragments, namely: bentonite, clinoptilolite and trepel, were studied. It was found that the selectivity of products depends on nature of catalyst and the reaction conditions, instead, in the presence of samples modified with nitric acid (H-samples), the main product is monoacetin. The main factors that allow regulating the activity of such catalysts and the efficiency of acetylation process are established. For example, the studied reaction refers to the processes of self-catalysis and in this case the molecule of acetic acid appear as a reactant and as a catalyst. The conversion of glycerol reaches 80 % and the main product is monoacetin (>60 %). Therefore, the main function of the catalyst in this reaction is to increase the selectivity of di- and triacetin. Regardless of the catalysts mass fraction, the glycerol conversion doesn´t change (~100 %), but changes depending on the reaction temperature and the mole ratio of reactants. According to their catalytic activity, the studied catalysts can be placed in following order: AS-Bent > AS-Tr > AS-CLI.
References
Perez, F. M.; Gatti, M. N.; Santori, G. F.; Pompeo, F. (2023). Transformations of glycerol into high-value-added chemical products: Ketalization and esterification reactions. Reactions, 4, 569-634. https://doi.org/10.3390/reactions4040034
Sandid, A., Spallina, V., Esteban, J. (2024). Glycerol to value-added chemicals: State of the art and advances in reaction engineering and kinetic modelling. Fuel Process. Technol., 253, 108008108057. https://doi.org/10.1016/j.fuproc.2023.108008
Almeida, E. L., Olivo, J. E., Andrade, C. M. G. (2023). Production of biofuels from glycerol from the biodiesel production process – A brief review. Fermentation, 9, 869–887. https://doi.org/10.3390/fermentation9100869
Liu, Y., Zhong, B., Lawa, A. (2022). Recovery and utilization of crude glycerol, a biodiesel byproduct. RSC Adv., 12(43), 27997–28008. https://doi.org/10.1039/D2RA05090K
Moklis, M. H.; Cheng, S.;Cross, J. S. (2023). Current and future trends for crude glycerol upgrading to high value-added products. Sustainability, 15, 2979–3009. https://doi.org/10.3390/su15042979
Rahman, H. (2023). Purifying crude glycerol from biodiesel production for sustainable energy solutions. J. Teknol., 11(1), 85–99. https://doi.org/10.31479/jtek.v11i1.269
Yadav, N., Yadav, G., Ahmaruzzaman, M. (2024). Catalytic conversion and mechanism of glycerol into various value-added products: A critical review. Ind. Crops Prod., 210, 11799. https://doi.org/10.1016/j.indcrop.2023.117999
Tabassum, N., Pothu, R., Pattnaik, A., Boddula, R., Balla, P., Gundeboyina, R., Challa, P., Rajesh, R., Perugopu, V., Mameda, N., Radwan A. B., Abdullah A. M., Al-Qahtani, N. (2022). Heterogeneous catalysts for conversion of biodiesel-waste glycerol into high-added-value chemicals. Catalysts, 12, 767–800. https://doi.org/10.3390/catal12070767
Liu, Y., Mai, C. T. Q., Ng, F. T. T. (2021). Glycerol hydrogenolysis with in situ hydrogen produced via methanol steam reforming: the promoting effect of Pd on a Cu/ZnO/Al2O3 catalyst. Catalysts, 11(1), 110–131. https://doi.org/10.3390/catal11010110
Medina, A., Abad, J. I., Tolvanen, P., de Araujo Filho, C., Salmi, T. (2022). Revisiting the kinetics and mechanism of glycerol hydrochlorination in the presence of homogeneous catalysts. Ind. Eng. Chem. Res., 61(37), 13827–13840. https://doi.org/10.1021/acs.iecr.2c01805
Hu, X., Lu, J., Liu, Y., Chen, L., Zhang, X., Wang, H. (2023). Sustainable catalytic oxidation of glycerol: a review. Environ. Chem. Lett., 21(5), 2825–2861. https://doi. org/10.1007/s10311-023-01608-z
Wang, H., Ma, J. (2024). Reaction kinetics and mechanism for the synthesis of glycerol carbonate from glycerol and urea using ZnSO4 as a catalyst. Catalysts, 14, 41–61. https://doi.org/10.3390/catal14010041
Perez, F. M., Gatti, M. N., Nichio, N. N., Pompeo, F. (2022). Bio-additives from glycerol acetylation with acetic acid: Chemical equilibrium model. Results Eng., 15, 100502. https://doi.org/10.1016/j.rineng.2022.100502
Manríquez-Ramírez, M.E., Trejo-Valdez, M., Castro, L.V., Ortiz-Islas, E. (2024). Acetylation of glycerol using acetic acid and heterogeneous MgO-KOH-based catalysts to produce acetins. Catal Lett, 154, 3294–3308. https://doi.org/10.1007/s10562-023-04556-z
Banu, I., Bumbac, G., Bombos, D., Velea, S., Gălan, A-M., Bozga, G. (2020). Glycerol acetylation with acetic acid over Purolite CT-275. Product yields and process kinetics. Renewable Energy, 148, 548–557. https://doi.org/10.1016/j.renene.2019.10.060
Dosuna-Rodríguez, I., Gaigneaux, E. M. (2012). Glycerol acetylation catalysed by ion exchange resins. Catal. Today, 195(1), 14–21. https://doi.org/10.1016/j.cattod.2012.04.031
Dewajani, H., Zamrudy, W., Saroso, H., Paramarta, S., Mulya, W. (2019). Conversion of crude glycerol from by-product biodiesel into bio-additive of fuel through acetylation reaction based on modified zeolite catalyst. ALCHEMY: Journal of Chemistry, 7(2), 46–52. https://doi.org/10.18860/al.v7i2.8193
Nda-Umar, U. I., Ramli, I. B., Muhamad, E. N., Azri, N., Amadi, U. F., Taufiq-Yap, Y. H. (2020). Influence of heterogeneous catalysts and reaction parameters on the acetylation of glycerol to acetin: A review. Appl. Sci., 10(20), 7155–7189. https://doi.org/10.3390/app10207155
Banu, I., Bozga, G., Bumbac, G., Vintila, A., Velea, S., Galan, A. M., Bombos, M., Blajan, O., Crucean, A. C. (2019). A kinetic study of glycerol esterification with acetic acid over a commercial Amberlyst-35 ion exchange resin. Rev. Chim., 70(7), 2325–2329. https://doi.org/10.37358/RC.19.7.7332
Tangestanifard, M., Ghaziaskar, H. S. (2017). Arenesulfonic acid-functionalized bentonite as catalyst in glycerol esterification with acetic acid. Catalysts, 7(7), 211–221. https://doi.org/10.3390/catal7070211
Rakytska, T. L., Kiose, T. O., Truba, A. S., Raskola, L. A. (2018). [Physico-chemical properties of natural sorbents and metal complex catalysts based on them]. Navchaljnyj posibnyk dlja studentiv khimichnogho fakuljtetu (in Ukrainian). https://dspace.onu.edu.ua/handle/123456789/30388
Davtian, A. S., Levchenko, O. O., Kamalov, G. L., Pavlo S. Yaremov, P. S., Kurmach, M. M. (2024). Natural aluminosilicates modified with arenesulfonic acid fragments as catalysts for acetalization of glycerol with cyclohexanone. Journal of Chemistry and Technologies, 32(3), 509–517 (in Ukrainian). https://doi.org/10.15421/jchemtech.v32i3.304786
Zhou, L., Al-Zaini, E., Adesina, A. A. (2013). Catalytic characteristics and parameters optimization of the glycerol acetylation over solid acid catalysts. Fuel, 103, 617–625. https://doi.org/10.1016/j.fuel.2012.05.042
Khayoon, M. S., Triwahyono, S., Hameed, B. H., Jalil, A. A. (2014). Improved production of fuel oxygenates via glycerol acetylation with acetic acid. Chem. Eng. J., 243, 473–484. https://doi.org/10.1016/j.cej.2014.01.027
Davtian, A. S., Chikhichin, D. G., Levchenko, O. O., Kamalov, G. L. (2024). Effect of the acidic treatment on the catalitic properties of natural aluminosilicates in glycerol acetylation. Theor. Exp. Chem., 6, 412–417. https://doi.org/10.1007/s11237-024-09800-0
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

