EFFECTS OF pH AND EXTENDED HEATING ON α-diCARBONYL COMPOUNDS PRODUCED IN THE D-GLUCOSE-GLYCINE MODEL SYSTEМ
DOI:
https://doi.org/10.15421/jchemtech.v33i2.323177Keywords:
the Maillard reaction, Maillard reaction model system, intermediate stage, α-dicarbonyl compounds (α-DCs), intermediates.Abstract
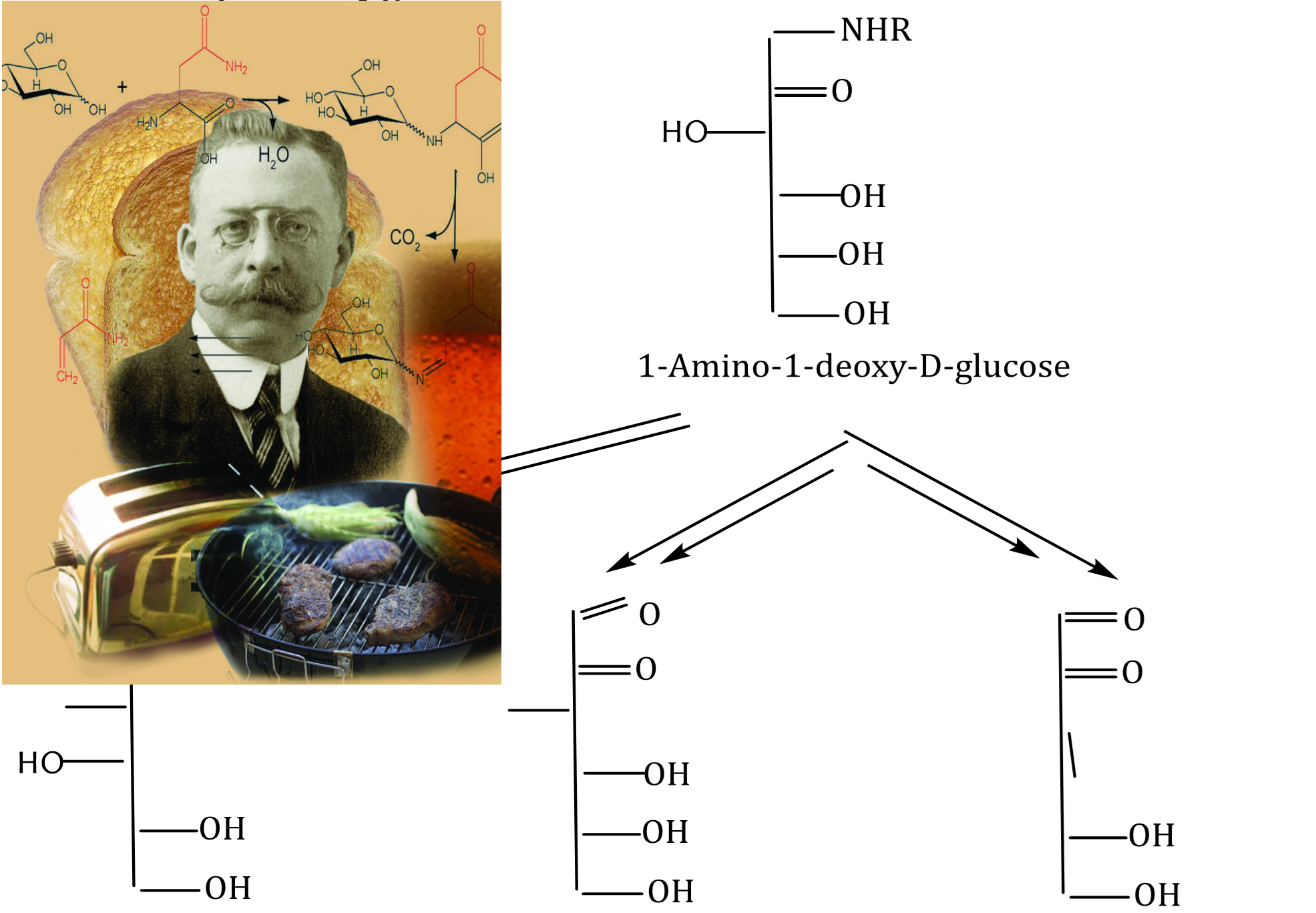
α-Dicarbonyl compounds (α-DCs) are significant markers in the intermediate stage of the Maillard reaction, which not only provide pleasant taste and color, extend shelf life and improve functionality, but also generate a large number of potentially harmful substances that can lead to severe food safety problems and pose significant risks to human health. Therefore, food derivatives of α-DCs are under close attention in food chemistry and medicine. The study of the effects of pH and extended heating on the formation of α-dicarbonyl compounds (α-DCs) in the D-glucose-glycine model system is important for understanding methods to reduce or prevent their development in heat-processed foods. In this study the α-DCs formation were determined based on the changes in UV absorbance at λmax 270–290 nm. The results indicated that in the pH range from 1.0 to 7.0 with increasing heating time the formation of α-DCs occurred most slowly in the pH range from 4.0 to 5.0. In the pH range from 7.0 to 12.0, the slower formation of α-dicarbonyl compounds was observed in the pH range from 7.0 to 10.0. The calculated relative rates of α-DCs formation in the D-glucose-glycine model system are in full agreement with the experimental data. The effect of both heating temperature and time on the formation of α-DCs in the D-glucose-glycine model system at pH 9 was investigated using Raman spectroscopy. The study of the α-DC formation patterns is to find the best and more suitable method for producing a healthy food product with good sensory characteristics.
References
Snelson, M., Melinda, T., Coughlan, M. T. (2019). Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients, 11(2), 215. doi: 10.3390/nu11020215.
Wei, X., Liu, L., Zhang, J., Kou, Y., Du, Y., Kong, M., Xie, J., Shen M. (2024). Evaluation of potentially harmful Maillard reaction products in different types of commercial formulae. Food Chemistry, 456, 139965. https://doi.org/10.1016/j.foodchem.2024.139965.
Shi, B., Guo, X., Liu, H., Jiang, K., Liu, L., Yan, N., Farag, M. A., Liu, L. (2024). Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chemistry, 438, 137994. doi: 10.1016/j.foodchem.2023.137994.
Dehghannya, J., Ngadi, M. (2021). Recent advances in microstructure characterization of fried foods: Different frying techniques and process modeling. Trends in Food Science & Technology, 116, 786–801. doi:10.1016/J.TIFS.2021.03.033.
Martins, F.C.O.L., Alcantara, G., Silva, A. F. S., Melchert, W.R., Rocha, F.R.P. (2022). The role of 5-hydroxymethylfurfural in food and recent advances in analytical methods. Food Chemistry, 395, 133539. https://doi.org/10.1016/j.foodchem.2022.13353
Zhang, C., Jia, T., He, J., Qi, H., Li, J., Xie, J. (2025). Effect of lysine on the cysteine-xylose Maillard reaction to form flavor compounds. Food Chemistry, 469, 142529. https://doi.org/10.1016/j.foodchem.2024.142529.
Xu, L., Liu, H., Dong, L., Liu, Y., Liu, L., Cao, H., Wang, W., Liu, К. (2024). Research advance on AGEs generation, detection, influencing factors and inhibition mechanism in bakery products processing. Food Bioscience, 57, 103404. https://doi.org/10.1016/j.fbio.2023.103404
Jia, W., Guo, A., Zhang, R., Shi, L. (2023). Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chemistry, 404 (A), 134541. https://doi.org/10.1016/j.foodchem.2022.13454
Meade, N., Maier, M. R. (2003). Evidence of long memory in short-term interest rates. Journal Metrics: Journal of Forecasting, 22(8), 553–568. https://doi.org/10.1002/for.873
Nooshkam, M., Varidi, M. (2024). Chapter Twelve - Antioxidant and antibrowning properties of Maillard reaction products in food and biological systems. Vitamins and Hormones, 125, 367–399. https://doi.org/10.1016/bs.vh.2024.01.001.
Kathuria, D., Hamid, G. S., Thakur, A. (2023). Maillard reaction in different food products: Effect on product quality, human health and mitigation strategies. Food Control, 153, 109911. https://doi.org/10.1016/j.foodcont.2023.109911.
Zhan, H., Tang, W., Cui, H., Hayat, K., Hussain, S., Tahir, M. U., Zhang, S., Zhang, X., Ho, C.-T. (2020). Formation kinetics of Maillard reaction intermediates from glycine–ribose system and improving Amadori rearrangement product through controlled thermal reaction and vacuum dehydration. Food Chemistry, 311, 125877. https://doi.org/10.1016/j.foodchem.2019.125877
Aktağ, A. G., Gökmen V. (2020). A survey of the occurrence of α-dicarbonyl compounds and 5-hydroxymethylfurfural in dried fruits, fruit juices, puree and concentrates. Journal of Food Composition and Analysis, 91, 103523. https://doi.org/10.1016/j.jfca.2020.103523 .
Shakoor, A., Zhang, C., Xie, J., Yang, X. (2022). Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chemistry, 393, 133416. https://doi.org/10.1016/j.foodchem.2022.133416.
Shi, B., Guo, X., Liu, H., Jiang, K., Liu, L., Yan, N., Farag, M. A., Liu, L. (2024). Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chemistry, 438, 137994. https://doi.org/10.1016/j.foodchem.2023.137994 .
Blidi, S., Troise, A.D., Zazzaroni, M., Pascale, S. De Cottin, S., Sturrock, K., Scaloni, A., Fiore, A. (2024). Effect of brewer's spent grain melanoidins on Maillard reaction products during storage of whey protein model systems. Current Research in Food Science, 8, 100767. https://doi.org/10.1016/j.crfs.2024.100767.
Li, Y., Sun, F., Xia, X., Liu, Q. (2025). Excessive oil absorption and maillard reaction products in fried muscle foods: Formation mechanisms, potential health risks and mitigation strategies. Food Chemistry, 468, 142456. https://doi.org/10.1016/j.foodchem.2024.142456
Huang, Z., Jiang, Y., Li, H., Li, Q., Gao, Z., Zhang, Y., Zhang, Q., Fu, L. (2023). Effect of glycation derived from α-dicarbonyl compounds on the in vitro digestibility of ovalbumin: Tracing of advanced glycation end-products and immuno-active peptides. Food Research International, 169, 112842. https://doi.org/10.1016/j.foodres.2023.112842.
Li, H., Ping, Y., Niranjan, K., Wu, Q., Chen, Z., Zhang, L., Zhao, B., Liu, K. (2024). Structure, antioxidant properties and AGEs (advanced glycation end products) formation of modified wheat gluten protein after enzymatic hydrolysis and Maillard reaction. Journal of Food Composition and Analysis, 136, 106795. https://doi.org/10.1016/j.jfca.2024.106795
Yuan, X., Feng, S., Li, J., Guo, R., Nie, C., Zhai, R., Tu, A., Cao, X., Zhang, M., Li, J. (2025). Generation of advanced glycation end products from glycated protein or fructose/glyoxal-protein adducts under in vitro simulated gastrointestinal digestion. Food Chemistry, 141175
Rabbani, N.,Thornalley, P. J. (2022). An Introduction to the Special Issue “Protein Glycation in Food, Nutrition, Health and Disease”. Intrernational Jornal of Molecular Sciences, 23(21), 13053. https://doi.org/10.3390/ijms232113053.
Brighina, S., Turrado, C. P., Restuccia, Cr., Walton, G., Fallic, O B., Oruna-Concha, M. J., Arena, E. (2021). Detrimental effect on the gut microbiota of 1,2-dicarbonyl compounds after in vitro gastro-intestinal and fermentative digestion. Food Chemistry, 341(1), 128237. https://doi.org/10.1016/j.foodchem.2020.128237
Kou, Y., Song, Z., Jing, Y., Li, H., Wei, X., Xie, J., Shen, M. (2024). Key Maillard intermediates - α-dicarbonyl compounds in foods: Occurrence, analysis, toxicity, and mitigation strategies. Food Control, 166, 110652. https://doi.org/10.1016/j.foodcont.2024.110652
Peng, H., Gao, Y., Zeng, C., Hua, R., Guo, Y., Wang, Y., Wang, Z. (2024). Effects of Maillard reaction and its product AGEs on aging and age-related diseases. Food Science and Human Wellness, 13(3), 1118–1134. https://doi.org/10.26599/FSHW.2022.9250094.
Fallavena, L. P., Rodrigues, N. P., Marczak, L.D.F., Mercali, G. D. (2022). Formation of advanced glycation end products by novel food processing technologies: A review. Food Chemistry, 393, 133338. https://doi.org/10.1016/j.foodchem.2022.133338
Chen, N., Xu, X., Yang, X., Hu, X., Chen, F., Zhu, Yu. (2025). Polyphenols as reactive carbonyl substances regulators: A comprehensive review of thermal processing hazards mitigation. Food Research International, 200, 115515. https://doi.org/10.1016/j.foodres.2024.115515
Yuan, X.-Y., Meng, C., Liu, H., Sun, B. (2023). Magnetically driven nanorobots based on peptides nanodots with tunable photoluminescence for rapid scavenging reactive α-dicarbonyl species and effective blocking of advanced glycation end products. Food Chemistry, 422, 136252. https://doi.org/10.1016/j.foodchem.2023.136252
He, J., Wang, L., Liu, H., Sun, B. (2024). Recent advances in molecularly imprinted polymers (MIPs) for visual recognition and inhibition of α-dicarbonyl compound-mediated Maillard reaction products, Food Chemistry, 446, 138839. https://doi.org/10.1016/j.foodchem.2024.138839
Rodriguez-Amaya, D. B., Amaya-Farfan, J. (2024).The Maillard reactions: Pathways, consequences, and control. Vitamins and Hormones, 125, 149–182. https://doi.org/10.1016/bs.vh.2024.04.002
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

