SYNTHESIS OF N-ACYLOXY-1-(DIMETHOXYPHOSPHORYLOXY)BENZIMIDATES FROM N-ACYLOXY-N-CHLOROBENZAMIDES
DOI:
https://doi.org/10.15421/jchemtech.v33i2.323425Keywords:
N-acyloxy-N-chlorobenzamides, trimethyl phosphite, N-acyloxy-1-(dimethoxyphosphoryloxy)benzimidates, synthesis, structure, XRD study, intramolecular N–O-migration of dimethoxyphosphoryl groupAbstract
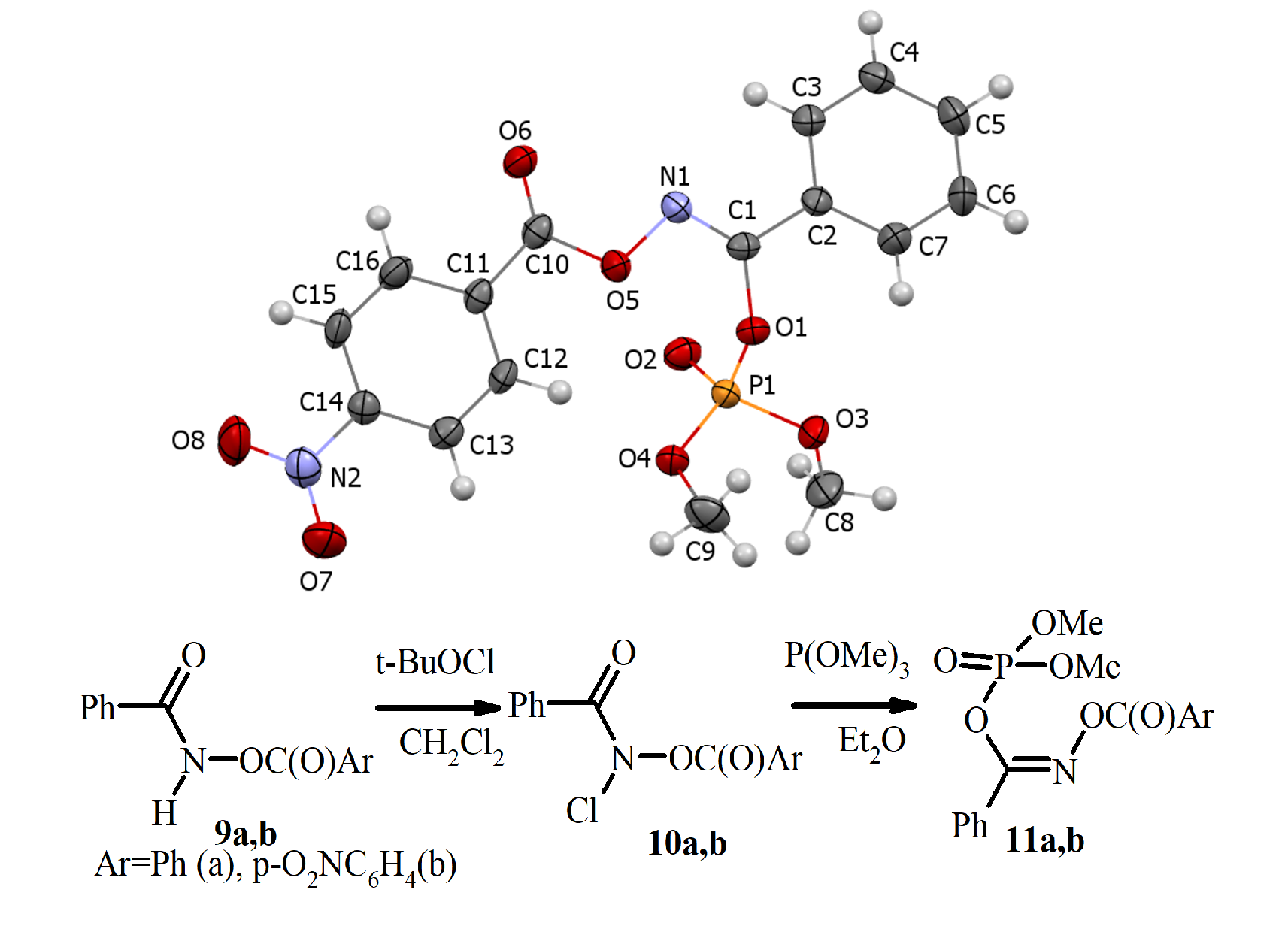
Aim. The objective of this research was to investigate the potential interaction between N-acyloxy-N-chlorobenzamides and trialkyl phosphites, along with the characterization of the resulting products' structures. Methods. Employing techniques such as 1H, 31P and 13C NMR spectroscopy, mass spectrometry, and single crystal X-ray diffraction, we have proved that the reaction of N-acyloxy-N-chlorobenzamides with trimethyl phosphite in diethyl ether produces N-acyloxy-1-(dimethoxyphosphoryloxy)benzimidates. Our research demonstrates that the reaction between N-acyloxy-N-chlorobenzamides and trialkyl phosphites offers a novel approach to synthesize Z-N-acyloxy-1-(dialkoxyphosphoryloxy)benzimidates. This discovery unveils a significant chemical transformation of N-acyloxy-N-chlorobenzamides. The structure of N-acyloxy-1-(dimethoxyphosphoryloxy)benzimidates has been confirmed by 1H, 31P and 13C NMR spectroscopy, mass spectrometry, and XRD study. The study of the N-(4-nitrobenzoyloxy)-1-(dimethoxyphosphoryloxy)benzimidate structure has revealed that the N-(4-nitrobenzoyloxy)-1-(dimethoxyphosphoryloxy)benzimidate is the Z-isomer, with the dimethoxyphosphoryloxy moiety and the N-4-nitrobenzoyloxy group being cis-oriented to the N=C double bond. The ether moiety and the N=C double bond are coplanar, while the dimethoxyphosphoryl substituent is orthogonal to the plane of the N=C double bond. The interaction of N-acyloxy-N-chlorobenzamides with trimethyl phosphite has led to a new synthesis of Z-N-acyloxy-1-(dialkoxyphosphoryloxy)benzimidates. The new chemical properties of N-acyloxy-N-chlorobenzamides have been established. The X-ray study of Z-N-4-nitrobenzoyoxy-1-benzimidate has demonstrated the peculiarities of its structure. Notably, an intriguing phenomenon of nitrogen-to-oxygen migration of the dimethoxyphosphoryl group has been observed.
References
Oeser, P., Tobrman, T. (2024). Organophosphates as Versatile Substrates in Organic Synthesis. Molecules, 29(7), 1593−1637. https://doi.org/10.3390/molecules29071593
Chrobak, E., Bebenek, E., Kadela-Tomanek, M., Latocha, M., Jelsch, C., Wenger, E., Boryczka, S. Betulin (2016). Phoshonates; Synthesis, Structure, and Cytotoxic Activity. Molecules, 21(9), 1123-1136. https://doi.org/10.3390/molecules21091123
Shtamburg, V. G., Klots, E. A., Shtamburg, V. V., Anishchenko, A. A., Shishkina, S. V., Mazepa, A.V., Kravchenko, S. V. (2024). Synthesis N-Alkoxy-1-(dimethoxyphosphoryloxy)benzimidates from N-Alkoxy-N-chlorobenzamides. J. of Chemistry and Technologies, 32(3), 528−537.https://doi.org/10.15421/jchemtech.v32i3.298733
Plapinger, R. E., Wagner-Jauregg, T. (1953). A Nitrogen-to-Oxygen Phosphoryl Migration: Preparation of dl-Serinephosphoric and Threoninephosphoric Acid. J. Am. Chem. Soc., 75(22), 5757–5758. https://doi.org/10.1021/ja01118a524
Gao, X., Deng, H., Tang, G., Liu, Y., Xu, P., Zhao, Y. (2011). Intermolecular Phosphoryl Transfer of N-Phosphoryl Amino Acids. Eur. J. Org. Chem., (17), 3220−3228. https://doi.org/10.1002/ejoc.201100234
Shtamburg, V. G., Klots, E. A., Shtamburg, V. V., Anishchenko, A. A., Shishkina, S. V., Mazepa, A. V. (2023). Nucleophilic substitution at nitrogen atom. N-Alkoxy-N-(dimethoxyphosphoryl)ureas, synthesis and structure. J. Mol. Structure, 1277, 134865.https://doi.org/10.1016/j.molstruc.2022.134865
Shtamburg, V. G., Klots, E. A., Anishchenko, A. A., Shishkina, S. V., Mazepa, A.V., Kravchenko, S. V. (2024). Interaction of N-Alkoxy-N-chloro derivatives of amides, sulfonamides and ureas with trialkyl phosphites. XXVI Ukrainian Conference of Organic Chemistry and Biochemistry, Uzhgorod, 16-20 September 2024, Thesis, D-5. (In Ukrainian)
Shtamburg, V. G., Klots, E. A., Shtamburg, V. V., Anishchenko, A. A., Shishkina, S. V., Kravchenko, S. V. Mazepa, A.V. (2025). Dialkyl-N-Alkoxy-N-(4-toluenesulfonyl)phosphoramidates: Synthesis and Structure. Voprosy khimii I khimicheskoi tecknologii – Issues of Chemistry and Chemical Technology, 2025(2), 33-44.
Kravchenko, S. V. (2013). N-Chloro-N-benzoyloxybenzamide. Bull. Dnipropetrovsk Univ., Ser. Chem., 21(19), 61−65.https://doi.org/10.15421/08132119
Sheldrick, G. M. (2008). A short history of SHELX. Acta Cryst., Sect. A., A 64, 112−122. https://doi.org/10.1107/S0108767307043930
Pu, X., Li, Q., Lu, Z., Yang, X. (2016). N-Chloro-N-methoxybenzenesulfonamide: A Chlorinating Reagent. Eur. J. Org. Chem., (36), 5937−5940. https://doi.org/10.1002/ejoc.201601226
Wang Yu, Bi C., Kawamata Yu, Grant L. N., Samp L., Richardson P. F., Zhang S., Harper K. C., Palkowitz M. D., Vasilopoulos, A., Collins M. R., Oderinde, M. S., Tyrol, C. C., Chen, D., LaChapelle, E. A., Bailey, J. B., Qiao, J. X., Baran, P. S. (2024). Discovery of N−X anomeric amides as electrophilic halogenation reagents. Nature Chemistry, 16, 1539−1545. https://doi.org/10.1038/s41557-024-01539-4
Glover, S. A. (1998). Anomeric Amides – Structure, Properties and Reactivity. Tetrahedron, 54(26), 7229–7271. https://doi.org/10.1016/S0040-4020(98)00197-5
Shtamburg, V. G., Klots, E. A., Pleshkova, A. P., Avramenko, V. I., Ivonin, S. P., Tsygankov, A.V., Kostyanovsky, R. G. (2003). Geminal systems. 50. Synthesis and alcoholysis on N-acyloxy-N-alkoxy derivatives of ureas, carbamates and benzamides. Russ. Chem. Bull., Int. Ed., 52(10), 2251−2260. https://doi.org/10.1023/B:RUCB.0000011887.40529.b0
Glover, S. A., Rosser, A. A. (2018). Heteroatom Substitution at Amide Nitrogen – Resonance Reduction and HERON Reactions of Anomeric Amides. Molecules, 23(11), 2834. https://doi.org/10.3390/molecules23112834
Glover, S. A. (2009). N-Heteroatom-substituted hydroxamic esters, in The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids. Eds Rappoport, Z., Liebman, J. F., John Wiley and Sons, New York. PATAI’S Chemistry of Funcional Groups, 839–923.https://doi.org/10.1002/9780470682531.pat0470
Shtamburg, V. G., Tsygankov, A. V., Shishkin, O. V., Zubatyuk, R. I., Uspensky, B. V., Shtamburg, V. V., Mazepa, A. V., Kostyanovsky, R .G. (2012). The properties and structure of N-chloro-N-methoxy-4-nitrobenzamide. Mendeleev Commun., 22(3), 164−166. https://doi.org/10.1016/j.mencom.2012.05.019.
Digianantonio, K. M., Glover, S. A., Johns, J .P., Rosser, A. A. (2011). Synthesis and termal decomposition of N,N-dialkoxyamides. Org. Biomol. Chem., 9, 4116−4126. https://doi.org./10.1039/C1OB00008J
Glover, S. A., White, J. M., Rosser, A. A., Digianantonio, K. M. (2011). Structure of N,N-Dialkoxyamides: Pyramidal Anomeric Amides with Low Amidicity. J. Org. Chem., 76 (23), 9757–9763. https://doi.org./10.1021/jo201856u
Glover, S. A., Rosser, A. A., Taherpour, A., Greatrex, B. (2014). Formation and HERON Reactivity of Cyclic N,N-Dialkoxyamides. Aust. J. Chem., 67(3), 507–520. https://doi.org./10.1071/CH13557
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

