STUDY OF KINETIC REGULARITIES OF HETEROCATALYTIC OXIDATION REACTIONS OF CHLOROHYDROCARBONS C6, C7
DOI:
https://doi.org/10.15421/jchemtech.v33i3.324490Keywords:
kinetic regularity, chlorotoluene, chlorobenzene, oxidation reaction, catalyst.Abstract
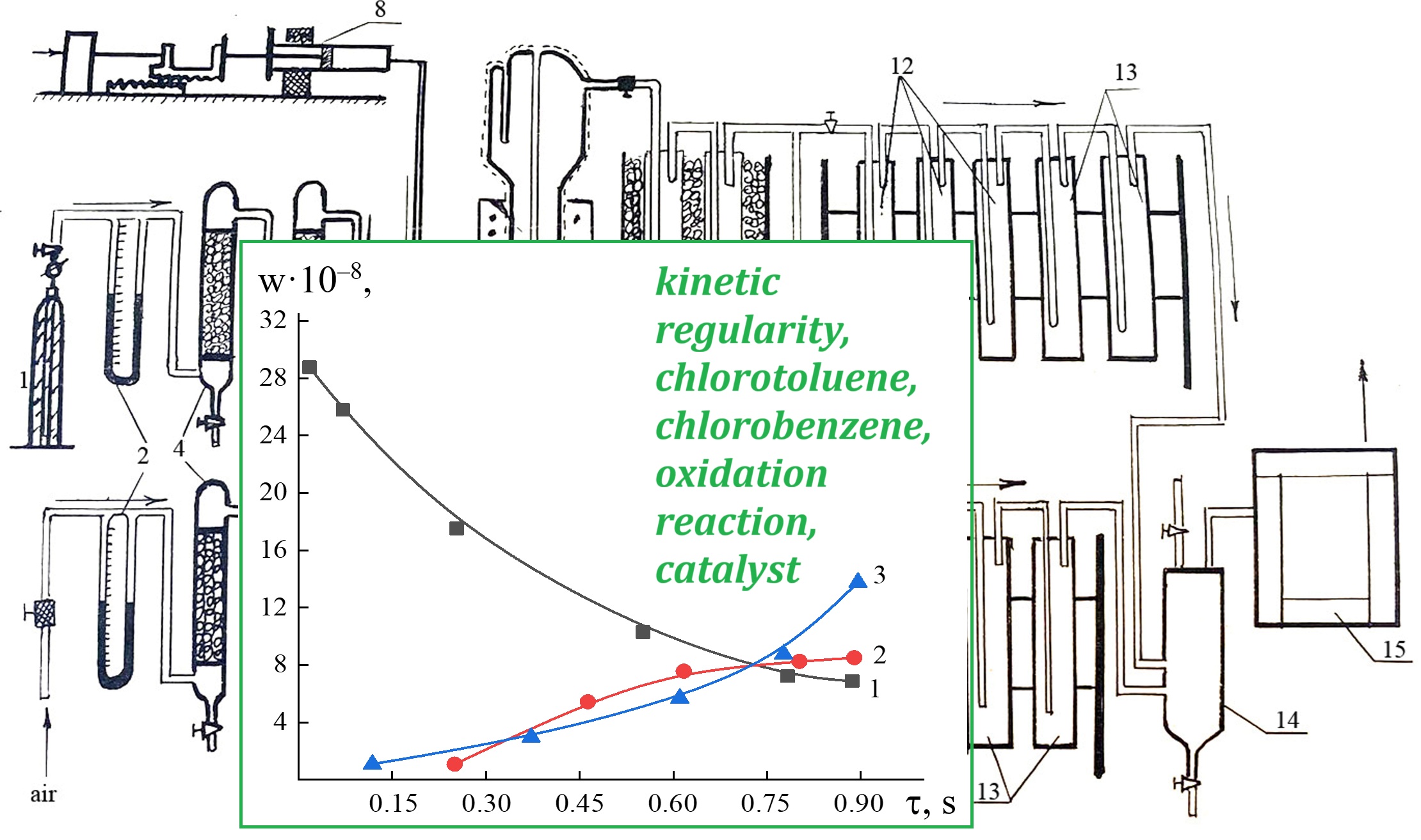
The kinetic regularities of the oxidation reaction of chlorotoluenes and chlorobenzenes on vanadium-containing catalysts have been studied, and on this basis, methods have been developed to control the activity and selectivity of ChCs (chlorohydrocarbons) oxidation processes. It has been shown that the oxidation rate of chlorohydrocarbons gradually increases with increasing temperature and contact time. It has been established that the oxidation processes of chlorohydrocarbons proceed through parallel-consecutive reactions with the formation of chlorine and dichloromaleic anhydrides and deep oxidation products. The presence, position and number of chlorine atoms affect the direction of the oxidation reaction. It has been shown that the rate of oxidation of chlorohydrocarbons to CO2 on a fixed catalyst bed is higher than on a fluidized bed, although the rate of formation of the targeted products on a fluidized bed is higher. It has been established that both the initial chlorohydrocarbons and their oxidation products are compactly adsorbed on the surface of vanadium-phosphorous catalysts. The reaction products are stable to decomposition and have a significant effect on the oxidation kinetics of chlorohydrocarbons. It has been revealed that the main end products (CO2, MCMA, DCMA), as well as some intermediate compounds, have a modifying effect on the active centers of the catalyst increasing the rate of selective oxidation of ChCs. It has been determined that the selective catalytic oxidation of CO occurs at ratios of CO : O2 = 1 : 15 and higher, high catalysis rates are also observed at these ratios. It has been shown that during the oxidation of chlorobenzenes, MCMA and DCMA are formed, whereas during the oxidation of chlorotoluenes, chlorobenzaldehyde is also formed in addition to the above-listed compounds. The water formed in the oxidation reaction of chlorobenzenes and chlorotoluenes has a positive effect on active catalysts up to a certain concentration, modifying the active centers.
References
Kiperman, S.A. (1964). Introduction to the kinetics of heterogeneous catalytic reactions. M.: Nauka, 514.
Li, X., Chen, Y., Chen, Z, Guo, H, Yang, S., Ma, X. (2022). The recent progress on gaseous chlorinated aromatics removal for environmental applications. Sep. Purif. Technol. 121364. https://doi.org/10.1016/j.seppur.2022.121364
Yang, L., Liu, P., Zhang, H.-Yu, Zhang, Y., Zhao, J. (2020). Catalytic Oxidation of o-Chlorotoluene with Oxygen to o-Chlorobenzaldehyde in a Microchannel Reactor. J. Ame. Chem. Soc. 24(10), 2034–2042, https://doi.org/10.1021/acs.oprd.0c00135
Jacques, V. (2016). Heterogeneous catalytic partial oxidation of lower alkanes (C1–C6) on mixed metal oxides. J. Energy Chem. Elsevier, 25(6), 936–946. DOI: 10.1016/j.jechem.2016.10.007
Melikova, I.G., Efendi, A.J., Aykan,N. F., Babayev E.M., Faradjev, G. M. and Rustamova, J. T. (2023). Catalysts and Kinetic Regularities of Oxidation Processes of Chlorotoluenes. Eurasian Journal of Chemistry , 4 (112), 134-140. https://doi.org/10.31489/2959-0663/4-23-3.
Dong, Y., Gao, S., Feng, J., Wang, X., Zhu, K., Wu, K., Guo, R. (2024). Recent advances in catalytic oxidation of chlorobenzene over metal oxide-based catalysts. Sep. Purif. Technol., 128098. https://doi.org/10.1016/j.seppur.2024.128098
Torsten Klement, Schirin Hanf, Fabian Wolff , Norbert Kockmann, Stephan A. Schunk and Thorsten Röder (2021). Oscillating droplet reactor – towards kinetic investigations in heterogeneous catalysis on a droplet scale. React. Chem. Eng., 6, 1023-1030. DOI: 10.1039/D0RE00466A
Melikova, I. H. (2016). Oxidation of chlorotoluene, toluene and chlorobenzene in the presence of Ag/MnO2 catalyst. Azerbaijan Oil Industry, 202(210), 47–52. doi: 10.37474/0365-8554/ 2022-10-47-52
Liu, J., Liu, L., Zhang, W., Li, P., Li, X., Yu, Z., Su, W. (2023). Continuous catalytic aerobic oxidation of o chlorotoluene to o-chlorobenzoic acid under slug flow conditions. J. Flow Chemistry, 13, 325–335. doi: 10.1007/s41981-023-00272-2
Melikova, I.H., Efendiyev, A.J., Yunisova, F.A. (2001). Reactivity of chlorohydrocarbons in catalytic oxidation reactions. Azerb. Chem. J., 2, 13–18.
Zhang, X., Hong, J., Zhang, Ch., Liu, Q., Zhang, J., Qian, G. (2022). Controlling effect of TiO2 carrier on formed vanadium species for effective catalytic oxidization of chlorobenzene. J. Applied Surface Science, 605, 154643. https://doi.org/10.1016/j.apsusc.2022.154643
Zhang, T. (2020). Heterogeneous Catalytic Process for Wastewater Treatment. Advanced Oxidation Processes. DOI: 10.5772/intechopen.90393
Dhakshinamoorthy, A., Alvaro, M., Garcia, H. (2011). Metal-organic frameworks as heterogeneous catalysts for oxidation reactions. Catal. Sci. Technol., 1(6), 856–867. https://doi.org/10.1039/C1CY00068C
Xie, R., Cao, J., Xie, X., Lei, D., Guo, K., Liu, H., Zeng, Y., Huang, H. (2020). Mechanistic insights into complete oxidation of chlorobenzene to CO2 via wet scrubber coupled with UV/PDS. Chem. Eng. J., 401, 126077. https://doi.org/10.1016/j.cej.2020.126077
Babayev, E., Efendi, A., Aykan, N. (2014). Oxidation reactions of chlorobenzene and chlorotoluene in the presence of oxide catalysts. World Forum of Young Scientists Collection of abstracts, 143–145.
Jacques, C.V. Heterogeneous Catalysis on Metal Oxides (2017). Catalysts, 7(11), 341.
https://doi.org/10.3390/catal7110341
Melikova, I. G., Efendi, A. J., Aykan, N. F., Babayev, E. M., Farajev, G. M., Rustamova, C. T. (2023). Catalysts and kinetic regularities of oxidation processes of chlorotoluenes. Eurasian Journal of Chemistry. 112(4), 130–140; DOI:10.31489/2959-0663/4-23-3
Qin, Y., Gu, J., Cai, W., Wang, Zh. (2022) Catalytic oxidation of chlorobenzene and PCDD/Fs over V2O5-WO3/TiO2: insights into the component effect and reaction mechanism. J. Environ Sci. Pollut. Res. Int., 29(28), 42809–42821. doi: 10.1007/s11356-022-18768-0.
Salehli, N.F., Efendi, A.J., Melikova, I.H. et al. (2004). Catalytic oxidation reactions of 1,3,5- and 1,2,4-trichlorobenzenes. Azerb. Chem. J. 4, 88-94
Li, W., Hu, D., Yin, K., Yu, Ch., Huang, B. (2022). Hierarchical Ru-Ce/H-ZSM-5 catalysts for the catalytic oxidation of chlorobenzene: Structure-activity relationship and chlorine poisoning resistance. J. Surfaces and Interfaces, 34, 102320
Zhou, W., Chen, L., Xie, J. (2015). Efficient synthesis of p-chlorobenzaldehyde through liquid-phase oxidation of p-chlorotoluene using manganese-containing ZSM-5 as catalyst, RSC Adv., 91, 74162–74169. https://doi.org/10.1039/C5RA16206H
Liu, J., Zhang, Y., Yan, X. Wang, Z., Wu, F., Fang, S., Jian, P. (2018). Selective oxidation of o‐chlorotoluene to o‐chlorobenzaldehyde catalyzed by (Co,Mn)(Co,Mn)2O4 catalysts. The Canadian Journal of Chemical Engineering.,96, 1746–1751. doi:10.1002/cjce.23116
Melikova, I.H., Aykan, N.F., Efendi, A.J., Babayev, E.M., Faradjev, H.M., Yunisova, F.A., Nebiyeva, M.F. (2022). Kinetic regularities of the reaction of catalytic oxidation of aromatic chlorohydrocarbons. Readings of A.I. Bulatov. Collection of Articles, 103–106.
Manafov, M.R. (2016). Software application for solving some typical problems of chemical technology. Azerb. Chem. J., 2, 89–94.
Manafov, M. R. (2015). Development of a Software Application for Solving of Problems of Chemical Kinetics and its Implementation in a C#. Int. J. Eng. Appl. Sci. 2(10), 33–37.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

