AGITATION LEACHING OF URANIUM FROM THE MICHURINSK DEPOSIT
DOI:
https://doi.org/10.15421/jchemtech.v33i1.325237Keywords:
Acid leaching, Uranium, Ferrous sulfate, Titanium white waste acid, oxidantAbstract
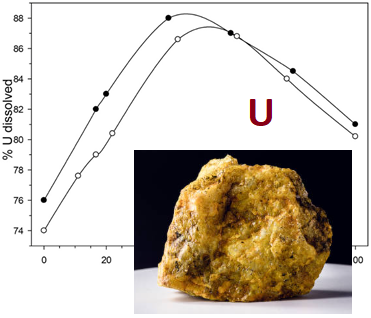
This research is dedicated to the investigation of agitation leaching of uranium ore from the Michurinsk deposit at 75 °C and atmospheric pressure, with the addition of ferric sulfate as a uranium oxidant in the composition of oxidized titanium white waste acid from titanium dioxide pigment production. It has been demonstrated that ferrous sulfate in titanium white waste acid solutions is quantitatively oxidized by oxygen to ferric sulfate in a gas-liquid reactor with mechanical agitation, using nitrogen compound-based catalysts within a time frame acceptable for the technological process. Replacing up to 40 % of sulfuric acid in uranium acid leaching solutions with oxidized titanium white waste acid allows for an increase in uranium extraction from 76 % to 88% at an Sol : Liq ratio of 1 : 1 and a total acid consumption of 90 kg/t of ore. The maximum uranium extraction corresponds to a leach filtrate concentration ratio of C(Fe³⁺)/C(Fe²⁺) ≥ 1. The use of counter-current agitation leaching in multiple stages can further enhance uranium extraction from refractory ore to above 90 %. However, substituting more than 40 % of sulfuric acid with oxidized titanium white waste acid leads to a decrease in uranium extraction due to the negative impact of impurities present in the waste acid. Increasing the consumption of sulfuric acid doped with oxidized titanium white waste acid to 110 kg/t of ore does not result in further uranium extraction improvement. The addition of oxidized titanium white waste acid in agitation leaching ensures a high level of uranium extraction from refractory ores. A final conclusion regarding the feasibility of implementing this approach in uranium concentrate production requires further investigation into the impact of impurities introduced with titanium white waste acid on other stages of the technological process.
References
Mihalasky, M., Michel-Sendis, F., Grancea, L. (2022). Uranium 2022: Resources, Production and Demand. NEA., 7634, 568.
Grancea, L., Mihalasky, M., Fairclough, M. (2020). Uranium Resources 2020, Production and Demand. NEA., 7551, 484.
Wang, B., Luo, Y., Li, X., Liu, Y., Xu, C., Zheng, Y., Zhang, Y., Zhou, Y., (2024). Water-Rock reactions in the acid leaching of Uranium: Hydrochemical characteristics and reaction mechanisms. J. Hydrol., 641, 131798. https://doi.org/10.1016/j.jhydrol.2024.131798.
Wang, J., Sun, Z., Li, G., Liu, Y., Zhou, Z., Wang, X., Zheng, Z., Zhou, Y., Zhao, K., Xiang, L., Wei, J. (2020). Geochemistry and Acid Hydrometallurgy Accessibility of Uraninite from Mianhuakeng Granite-Hosted Uranium Deposit, South China. Minerals, 10(9), 747. https://doi.org/10.3390/min10090747
Manfredi, C., Caruso, V., Vasca, E., Vero, S., Ventimiglia, E., Palladino, G., Ferri, D. (2006). On the Hydrolysis of the Tetravalent Uranium Ion U4+ . J. Solution Chem. 35, 927–937. https://doi.org/10.1007/s10953-006-9037-x
Habashi, F. (2020). Dissolution of uraninite. Hydrometallurgy, 194, 105329. https://doi.org/10.1016/j.hydromet.2020.105329
Mkhatshwa, S. F., Guy, B. M., Smith, A. J. B., Viljoen, K. S. (2020). A mineralogical perspective on the recovery of uranium from brannerite-rich ore at Cooke Section, West Rand Goldfield, South Africa. South African Journal of Geology, 123(4), 615–632. https://doi.org/10.25131/sajg.123.0031
Lin, H., Szenknect, S., Mesbah, A., Baron, F., Beaufort, D., Batonneau, Y., Mercadier, J., Eglinger, A., Turuani, M., Seydoux-Guillaume, A. M., Goncalves, P., Choulet, F., Chapon, V., Pagel, M., Dacheux, N. (2021). A multiparametric study on the dissolution of synthetic brannerite. npj Mater. Degrad., 5(1), 30. https://doi.org/10.1038/s41529-021-00173-6
Lottering, M. J., Lorenzen, L., Phala, N. S., Smit, J. T., Schalkwyk, G. A. C. (2008). Mineralogy and uranium leaching response of low grade South African ores. Miner. Eng., 21(1), 16–22. https://doi.org/10.1016/j.mineng.2007.06.006
Venter, R., Boylett, M. (2009). The evaluation of various oxidants used in acid leaching of uranium. J. South. Afr. Inst. Min. Metall., 445–446.
Ram, R., Charalambous, F., Tardio, J., Bhargav, S. (2011). An investigation on the effects of Fe (FeIII, FeII) and oxidation reduction potential on the dissolution of synthetic uraninite (UO2). Hydrometallurgy, 109(1–2), 125–130. https://doi.org/10.1016/j.hydromet.2011.06.005
Li, G., Yao, J., (2024). A Review of In Situ Leaching (ISL) for Uranium Mining. Mining, 4(1), 120–148. https://doi.org/10.3390/mining4010009
Bhargava, S. K., Ram, R., Pownceby, M., Grocott, S., Ring, B., Tardio, J., Jones, L. (2015). A review of acid leaching of uraninite. Hydrometallurgy, 151, 10–24. https://doi.org/10.1016/j.hydromet.2014.10.015
Ho, E. M., Quan, C. H. (2007). Iron(II) oxidation by SO2/O2 for use in uranium leaching. Hydrometallurgy, 85(2–4), 183–192. https://doi.org/10.1016/j.hydromet.2006.09.002
Wang, P., Tan, K., Li, Y., Liu, Z., Li, C., Tan, W., Tian, Y., Huang, W. (2022). Effect of Pyrite on the Leaching Kinetics of Pitchblende in the Process of Acid In Situ Leaching of Uranium. Minerals, 12(5), 570. https://doi.org/10.3390/min12050570
Zhang, Z., Li, J., Li, H., Guo, J., Chen, Y., Su, X., Hua, R. (2023) Research on conventional leaching process and leaching kinetics of a hard rock uranium mine. J. Radioanal. Nucl. Chem., 332, 4929–4942. https://doi.org/10.1007/s10967-023-09234-3
Kaksonen, A. H., Lakaniemi, A-M., Tuovinen O. H. (2020). Acid and ferric sulfate bioleaching of uranium ores: A review. J. Cleaner Prod., 264, 121586. https://doi.org/10.1016/j.jclepro.2020.121586
Epstein, I. R., Kustin, K., Warshaw, L. J. (1980). A kinetics study of the oxidation of iron (ΙΙ) by nitric acid. J. Am. Chem. Soc., 102(11), 3751–3758. http://doi.org/10.1021/ja00531a015
Gázquez, M.J., Contreras, M., Pérez -Moreno, S. M., Guerrero, J. L., Casas-Ruiz, M., Bolivar, J. P. (2021). A Review of the Commercial Uses of Sulphate Minerals from the Titanium Dioxide Pigment Industry: The Case of Huelva (Spain). Minerals, 11(6), 575. https://doi.org/10.3390/min11060575
Filippou, D., Hudon, G. (2009). Iron removal and recovery in the titanium dioxide feedstock and pigment industries. JOM 61, 36–42. https://doi.org/10.1007/s11837-009-0150-3
Zhou, Y., Li, G., Xu, L., Liu, J., Sun, Z., Shi, W. (2020). Uranium recovery from sandstone-type uranium deposit by acid in-situ leaching - an example from the Kujieertai. Hydrometallurgy, 191, 105209. https://doi.org/10.1016/j.hydromet.2019.105209
Gok, O. (2011). Catalytic oxidation mechanism of oxy-nitrogen spicies (NOx) in FeSO4 electrolyte. Nitric Oxide, 25(1), 47–53. https://doi.org/10.1016/j.niox.2011.05.003
(2000). [Technological Regulations of the Hydrometallurgical Plant of SE “SkhidGZK”] (In Ukrainian).
Nikiforova, A., Kozhura, O., Pasenko, O. (2017). Application of lime in two-stage purification of leaching solution of spent vanadium catalysts for sulfuric acid production. Hydrometallurgy, 172, 51–59. http://doi.org/10.1016/j.hydromet.2017.06.020
Gok, O. (2012). Ferrous oxidation catalyzed by oxy-nitrogen species (NOX). Asian J. Chem. 12, 5485–5489.
Laxen, P. A. (1971). Dissolution of uranium dioxide as an electron transfer reaction. the recovery of uranium. Proceedings of a Symposium. International Atomic Energy Agency, Vienna.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

