SYNTHESIS AND STUDY OF PHSYSICO-CHEMICAL PROPERTIES OF OLIGO(POLY)-MER SORBENTS BASED ON UROTROPINE AND CYANURIC ACID
DOI:
https://doi.org/10.15421/jchemtech.v33i2.325935Keywords:
urotropine, cyanuric acid, oligomeric sorbents, polymeric sorbents, adsorption, environmental applicationsAbstract
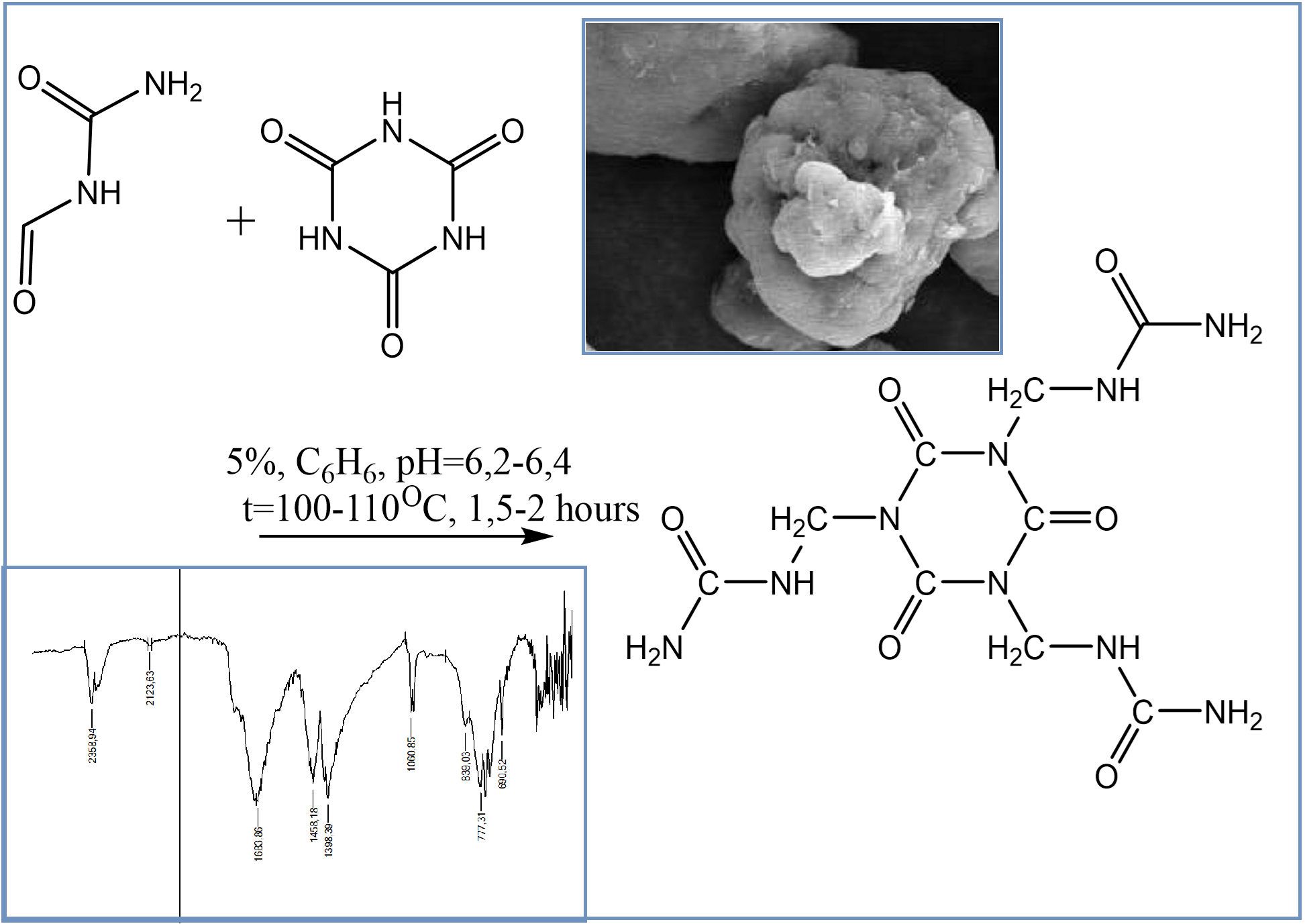
This study presents the synthesis and comprehensive physicochemical analysis of novel oligo(poly)mer sorbents derived from urotropine and cyanuric acid. The sorbents were obtained via controlled polycondensation reactions involving urea-formaldehyde systems modified with cyanuric acid, leading to structurally robust and functionally active oligomers. Advanced characterization techniques—including SEM, XRD, IR spectroscopy, and thermal analysis—confirmed the formation of new materials with unique morphology, crystallinity, and thermal stability. The synthesized CA+UFO(III) sorbents demonstrated high sorption efficiency for various 3d-metal ions (Cu²⁺, Ag⁺, Fe²⁺, etc.), with optimal performance in the pH range of 4–6. Results indicated that cyanuric acid not only enhances the reactivity of methylol groups but also stabilizes the oligomer structure, reducing formaldehyde release during storage. These findings highlight the potential application of CA-modified sorbents in environmental remediation and industrial wastewater treatment, offering an effective, tunable, and eco-friendly approach to selective metal ion adsorption.
References
Penchah, H. R. (2024). Functionalized and improved polymeric adsorbents. Polymeric Adsorbents. 159–176. https://doi.org/10.1016/B978-0-323-99746-1.00008-2
Golikand, A. N., Didehban, K.,Irannejad, L. (2012). Synthesis and characterization of triazine‐based dendrimers and their application in metal ion adsorption. Journal of Applied Polymer Science, 123(2), 1245–1251. https://doi.org/10.1002/app.33893
Peter, S. E., Thomas, P., Vairavel, P., Kumar, N. A. (2024). Cyanuric chloride as a linker towards the synthesis of covalent triazine polymers: a review. Materials Advances, 5(23), 9175–9209. https://doi.org/10.1039/d4ma00739e
Turaev, K., Umirova, G., Kasimov, S., Kobilova, M. (2023). Synthesis and studying of nitrogen and oxygen-containing complexing sorbents during the sorption of some d-metals, Science and innovation. 2(A11). 277–284.
Song, K. S., Fritz, P. W., Coskun, A. (2022). Porous organic polymers for CO2 capture, separation and conversion. Chemical Society Reviews, 51(23), 9831–9852. https://doi.org/10.1039/d2cs00727d
Du, J., Li, W. C., Ren, Z. X., Guo, L. P., Lu, A. H. (2020). Synthesis of mechanically robust porous carbon monoliths for CO2 adsorption and separation. Journal of Energy Chemistry, 42, 56–61. https://doi.org/10.1016/j.jechem.2019.06.006
Rovina K., Siddiquee S. (2016). Analytical and advanced methods-based determination of melamine, Nanobiosensors, 8, 339.
Gus’kov, V.Y., Gainullina, Y.Y., Musina, R.I., Zaripova, A.I., Pavlova, I.N. (2021). The emergence of chirality in cyanuric acid conglomerates by Viedma ripening: Surface characterization, chirality assessment and applications in chromatography. Separation Science and Technology, 56(3), 527–540. https://doi.org/10.1080/01496395.2020.1723030
Sun, M., Han, S., Feng, J., Li, C., Ji, X., Sun, H. (2021). Recent Advances of Triazine-Based Materials for Adsorbent Based Extraction Techniques. Topics in Current Chemistry (Cham), 379(4), 24–24. https://doi.org/10.1007/s41061-021-00336-8
Mao, H., Tang, J., Day, G. S., Peng, Y., Wang, H., Xiao, X., Reimer, J. A. (2022). A scalable solid-state nanoporous network with atomic-level interaction design for carbon dioxide capture. Science advances, 8(31), eabo6849. https://doi.org/10.1126/sciadv.abo6849
Yivlik, Y., Kizilcan, N., Akar, A. (2020). Isocyanuric acid-modified cyclohexanone–formaldehyde resins for fire-retardant polyurethane. Pigment & Resin Technology, 49(2), 119–126. https://doi.org/10.1108/PRT-03-2019-0025
Kholmurodova, S., Turaev, K. K., Alikulov, R., Beknazarov, K., Nomozov, A., &Eshmurodov, E. K. (2025). Obtaining an organic-inorganic sorbent based on vermiculite modified with urotropin and hydrolyzed polyacrylonitrile. Chemical Review and Letters, 8(2), 267–279. https://doi.org/10.22034/crl.2025.481061.1435
Nomozov, A.K. Turaev, K. K., Geldiev, Y., Kulbasheva, K.K., Yulchieva, M. G., Umirova, G., Toshtemirov, A., Khaitova, J. (2025). A review: synthesis of chelate-forming polymer ligands and their coordination compounds with d-metals. Chemical Review and Letters. https://doi.org/10.22034/crl.2025.507427.1547
Ahatov, A.A., Turaev,Kh.Kh., Tillaev,Kh.R., Kasimov, Sh.A, Nomozov, A.K. (2025). The Studying Synthesis of a New Polymer Sorbent based on O-phenylenediamine and Epichlorohydrin and Its Sorption Properties: Studying Synthesis of a New Polymer Sorbent based on O-phenylenediamine and Epichlorohydrin and Its Sorption Properties. Indian Journal of Chemistry, (IJC), 64(4), 353–360. https://doi.org/10.56042/ijc.v64i4.11686
Ermuratova, N.A., Turaev, K.K., Kornilov, K.N., Abduvalieva, M.Z., Chorieva, N.B. (2023). Adsorption Ability of Nitrogen-Containing Polymer Sorbents Based on Urea-Formaldehyde Resin and Aminoacetic Acid Towards Heavy Metal Ions. Polymer Science, Series A, 65(6), 666–671. https://doi.org/10.1134/S0965545X23600679
Buğdaycı, T., Bektaş, S., Akgül, E. T., Korkmaz, B., Yavuz, E., Senkal, B. F. (2023). Urethane-and urea-modified polymeric sorbents enable efficient and selective removal of mercury (II) from water. Polymer Bulletin, 80(11), 12079–12102. https://doi.org/10.1007/s00289-022-04642-z
Yulchieva, M.G., Turaev, Kh.Kh., Nomozov, A.K., Tovoshareva, I.E. (2024) A studying synthesis of a chelate-forming sorbent based on urea-formaldehyde and diphenylcarbazone: studying synthesis of a chelate-forming sorbent based on urea-formaldehyde and diphenylcarbazone. Indian Journal of Chemistry (IJC). 63.6, 579–585. https://doi.org/10.56042/ijc.v63i6.9006
Abduvalieva, M.J., Turaev, Kh.Kh., Kasimov,Sh.A. (2025) Complexing properties of ionite-polymer sorbent based on urea, formaldehyde and phenolsulfophthaleic acid. Azerbaijan Chemical Journal, 1, 127–138. https://doi.org/10.32737/0005-2531-2025-1-127-138.
Yulchieva, M. G., Turaev, Kh. Kh., Kasimov, Sh. A., Nabiev, D.A., Chorieva, N.B. (2023). Research on the synthesis of nitrogen-containing sorbents. Int. J. Eng. Trends Tech., 71, 161–167. https://doi.org/10.14445/22315381/IJETTV71I8P214
Liao, L., Li, M., Yin, Y., Chen, J., Zhong, Q., Du, R., Zeng, F. (2023). Advances in the synthesis of covalent triazine frameworks. ACS omega, 8(5), 4527–4542. https://doi.org/10.1021/acsomega.2c06961
Amrullaev, A., Boltaeva, S., Rashitova, S., Ganiev, B. (2024). Synthesis and study sorption properties oligo (poly)-mer sorbents based on urea-formaldehyde and cyanuric acid. In BIO Web of Conferences, 130, 06004. https://doi.org/10.1051/bioconf/202413006004
Zhang, L., Wu, Y., Liu, Y., Qu, Y. (2018). Adsorption mechanisms of metal ions on the potassium dihydrogen phosphate (1 0 0) surface: A density functional theory-based investigation. Journal of colloid and interface science, 522, 256-263. https://doi.org/10.1016/ j.jcis.2018.03.073.
She, D. M., Yu, H. L., Huang, Q. L., Li, F. M., Li, C. J. (2010). Liquid-phase synthesis of cyanuric acid from urea. Molecules, 15(3), 1898–1902. https://doi.org/10.3390/molecules15031898
Ghosh, K., Datta, M., Fröhlich, R., Ganguly, N. C. (2005). Urotropine: a unique scaffold in molecular recognition for phenolic substrates. Journal of molecular structure, 737(2-3), 201–206. https://doi.org/10.1016/j.molstruc.2004.10.019
Ting, Z., Min, Z., Xiao‐Mei, T., Feng, C., Jian‐Hui, Q. (2010). Optimal preparation and characterization of poly (urea–formaldehyde) microcapsules. Journal of applied polymer science, 115(4), 2162–2169. https://doi.org/10.1002/app.31329
(2013). EVO Scanning electron microscope Original instructions Carl Zeiss Microscopy, GmbH Carl-Zeiss-Promenade 1007745 Jena. – Germany.
Wang, M., Chang, X., Wu, X., Yan, H., Qiao, F. (2016). Water-compatible dummy molecularly imprinted resin prepared in aqueous solution for green miniaturized solid-phase extraction of plant growth regulators. Journal of Chromatography A, 1458, 9–17.
Haines, P. J. (2012). Thermal methods of analysis: principles, applications and problems. Springer Science & Business Media. XII, 286. https://doi.org/10.1007/978-94-011-1324-3
Nazarov, S., Razzokov, K., Shirinov, G., Niyozov, E., Rashidova, R., Rasulov, M., Ganiev, B. (2023). Investigation of thermal properties and composition on basalts of the Aydarkul deposit by methods DTA/DTG and X-ray diffraction. In E3S Web of Conferences, 389, 01023. https://doi.org/10.1051/e3sconf/202338901023
Amonov, M. R., Niyozov, E. D., Amonova, M. M., Nazarov, S. I., Ganiev, B. S. (2023). Study of chemical properties combination chemical method of wastewater treatment by methods IR-spectroscopy and X-ray diffraction. In E3S Web of Conferences 389, 01020. https://doi.org/10.1051/e3sconf/202338901020
Nazarov, S., Amrieva, S., Ganiev, B., Nazarov, N. (2024). Synthesis and spectroscopic study of adhesive polymer materials based on urea-formaldehyde and isoamyl alcohol. In BIO Web of Conferences, 130, 06003. https://doi.org/10.1051/bioconf/202413006003
GOST 14231-69 Urea-formaldehyde resins UKS I M19-62 (ru)
Feng, Y. (2014). A Method for Preparation of Resole Containing Low Methylol Group. Research of Materials Science, 3(4). 69-73. http://www.ivypub.org/RMS/en/ paperinfo/21723.shtml
Pedireddi, V. R., Belhekar, D. (2002). Investigation of some layered structures of cyanuric acid. Tetrahedron, 58(15), 2937–2941. https://doi.org/10.1016/S0040-4020(02)00202-8
Ganiev, B., Mardonov, U., Kholikova, G. (2023). Molecular structure, HOMO-LUMO, MEP-–Analysis of triazine compounds using DFT (B3LYP) calculations. Materials Today: Proceedings. https://doi.org/10.1016/j.matpr.2023.09.191
Xiong, C., Wang, H., Zhang, Z., Liang, K., Wu, C., Wu, W., Chen, Q. (2023). Triazine-based sulphur-containing polymers for Hg2+ adsorption: Efficiency and mechanism. Polymer, 289, 126480. https://doi.org/10.1016/j.polymer.2023.126480
Sun, Z., Yuan, F., Li, X., Li, C., Xu, J., Wang, B. (2018). Fabrication of novel cyanuric acid modified g-C3N4/kaolinite composite with enhanced visible light-driven photocatalytic activity. Minerals, 8(10), 437. https://doi.org/10.3390/min8100437
Pourebrahimi, S., Pirooz, M., De Visscher, A., Peslherbe, G. H. (2022). Highly efficient and reversible iodine capture utilizing amorphous conjugated covalent triazine-based porous polymers: Experimental and computational studies. Journal of Environmental Chemical Engineering, 10(3), 107805. https://doi.org/10.1016/j.jece.2022.107805
Gauglitz, G., Vo-Dinh, T. (Eds.). (2006). Handbook of spectroscopy. John Wiley & Sons. 600 p.
Ramazanov, B., Juraeva, L., Sharipova, N. (2021, September). Synthesis of modified amino-aldehyde oligo (poly) mers and study of their thermal stability. In IOP Conference Series: Earth and Environmental Science. 839(4), 042096. https://dois.org/10.1088/1755-1315/839/4/042096
Lee, H. (2023) Casein-based Medium Density Fibreboardusing Formaldehyde-free Binders: diss. The University of Auckland, New Zealand.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

