SYNTHESIS OF NITRIFICATION INHIBITORS BASED ON Mn(II) COORDINATION COMPOUNDS
DOI:
https://doi.org/10.15421/jchemtech.v33i4.328637Keywords:
ammonium, manganese, dicyandiamide, nitrate, nitrification, coordination compound, 4-amino-1,2,4-triazoleAbstract
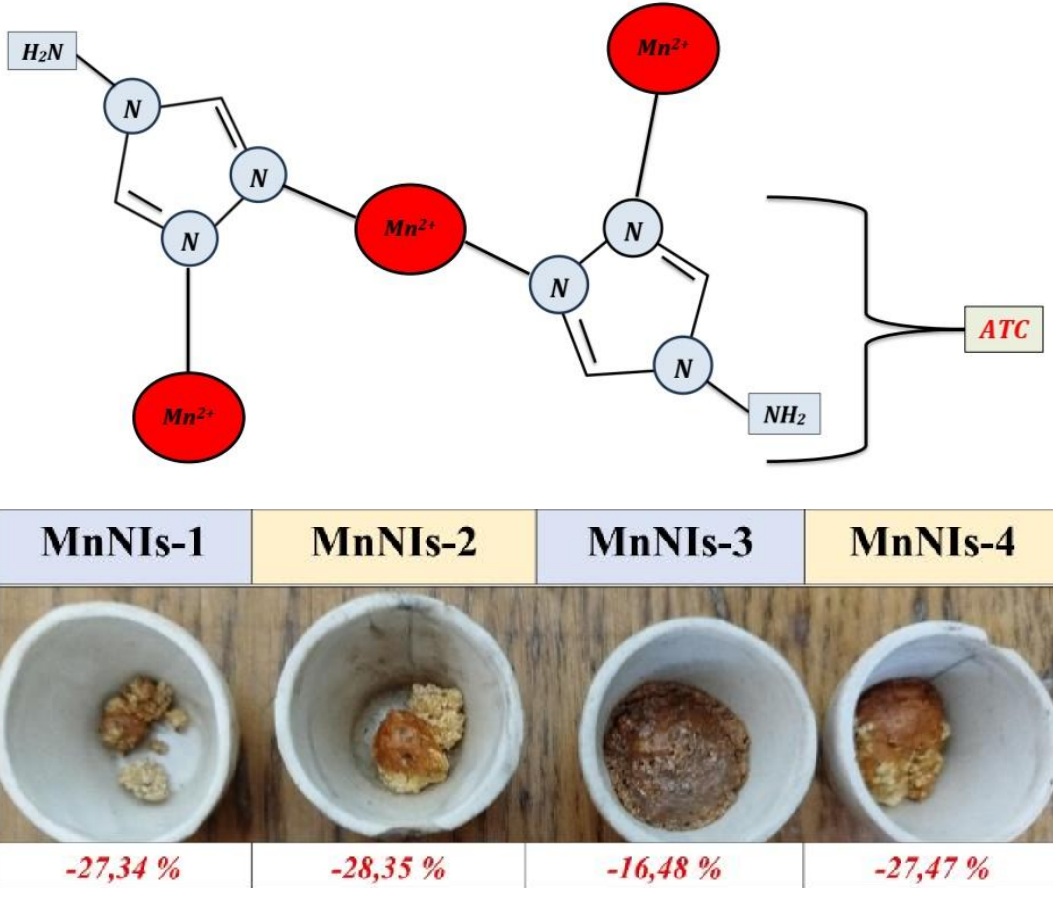
The combination of two effective nitrification inhibitors, such as dicyandiamide (DCD) and 4-amino-1,2,4-triazole (ATC) in manganese coordination compounds can exhibit a synergistic effect in relation to the nitrification process of ammonium nitrogen in the soil. As a result of this effect, the efficiency of nitrogen fertilizer use is predicted to increase. Manganese, as a biometal, after the end of the inhibitor action can be involved in biochemical reactions of crop development. Therefore, the aim of the work was the synthesis of nitrification inhibitors (NIs) based on Mn(II) coordination compounds. A number of studies of the structure, composition and biological activity of the synthesized compounds were carried out. As a result, four substances with a ligand ratio of 4-amino-1,2,4-triazole and dicyandiamide (1 : 1, 2 : 1, 1 : 2, 2 : 2) were obtained. Their solubility in water, aqueous solution of fertilizer UAN-28 and pure KAS-28 was established. Thermal destruction of the studied substances was studied. Infrared spectroscopy proved 1,2-coordination of 4-amino-1,2,4-triazole through nitrogen atoms. The addition of dicyandiamide is carried out through the C≡N functional group. The manganese content in complex compounds was established. The degree of nitrification inhibition and the dynamics of nitrification rate changes were studied in laboratory conditions.
References
Fathi, A. (2022). Role of nitrogen (N) in plant growth, photosynthesis pigments, and N use efficiency: A review. Agrisost, 28, e3917. https://doi.org/10.5281/zenodo.7143588
Jiaying, M., Tingting, C., Jie, L., Weimeng, F., Baohua, F., Guangyan, L., Hubo, L., Juncai, L., Zhihai, W. Longxing, T., Guanfu, F. (2022). Functions of Nitrogen, Phosphorus and Potassium in Energy Status and Their Influences on Rice Growth and Development. Rice Science, 29(2), 166–178. https://doi.org/10.1016/j.rsci.2022.01.005.
Guo, C., Wang, H., Zou, D., Wang, Y., Han, X. (2022). A novel amended nitrification inhibitor confers an enhanced suppression role in the nitrification of ammonium in soil. Journal of Soils and Sediments, 22, 831-843. https://doi.org/ 10.1007/s11368-021-03118-3
Yin, M., Gao, X., Kuang, W., Zhang, Y. (2023). Meta-analysis of the effect of nitrification inhibitors on the abundance and community structure of N2O-related functional genes in agricultural soils. Science of The Total Environment, 12(7), 1–13. https://10.1016/j.scitotenv.2022.161215
Fan, D., He, W., Smith, W.N., Drury, C.F., Jiang, R., Grant, B.B., Shi, Y., Song, D., Chen, Y., Wang, X., He, P., Zou, G. (2022). Global evaluation of inhibitor impacts on ammonia and nitrous oxide emissions from agricultural soils: A meta-analysis. Global Change Biology, 28(17), 5121-5141. https://doi.org/10.1111/gcb.16294
Li, J., Wang, W., Wang, W., Li, Y. (2022). The Ability of Nitrification Inhibitors to Decrease Denitrification Rates in an Arable Soil. Agronomy, 12(11), 2749. https://doi.org/10.3390/agronomy12112749
Malook, M.V., Matrosov, O.S., Rula, I.V. (2023). Complex zinc(II) compounds as nitrification inhibitors. Voprosy khimii i khimicheskoi tekhnologii, 6(151), 129-139. http://dx.doi.org/10.32434/0321-4095-2023-151-6-129-139
Trunova, O.K. (2023). The role of chelate coordination compounds of biogenic metals in the vital activity of plants. Ukrainian Chemistry Journa, 88(12), 91–138. https://doi.org/10.33609/2708-129X.88.12.2022.91-138
Malook, M.V., Matrosov, O.S., Vlasenko, К., Kuznetsova O.V. (2024). Preparation and properties of cobalt-containing nitrification inhibitors. Journal of Chemistry and Technologies, 32(2), 434–443. https://doi.org/10.15421/jchemtech.v32i2.297927
He, Т., Xie, D., Ni J., Li, Z., Li, Z. (2020). Effect of Cobalt, Cadmium and Manganese on Nitrogen Removal Capacity of Arthrobacter arilaitensis Y-10. Water, 12(1701), 1–12. https://doi.org/10.3390/w12061701
Kapustyan, A., Cherno, N. (2017). Chelate forms of biometalls. Theoretical aspects of obtaining and characteristics. Food Science and Technology, 11(1), 37–49. https://doi.org/10.15673/fst.v11i1.297
Bourdauducq, P., assignee Elf Atochem, S.A. (2003). United States Patent No. 6504033 (B1).
Ning, J., Ai, S., Cui, L. (2018). Dicyandiamidehas more inhibitory activities on nitrification thanthiosulfate. PLoS ONE, 13(8), 1–18. https://doi.org/10.1371/journal.pone.0200598
Taggert, B.I., Walker, C., Chen, D., Wille, U. (2021). Substituted 1,2,3-triazoles: a new class of nitrification inhibitors. Scientific Reports, 11(1), 1-12. https://doi.org/10.1038/s41598-021-94306-1
Pandey, R. (2015) Mineral Nutrition of Plants. Plant Biology and Biotechnology, 1, 499-538. https://doi.org/10.1007/978-81-322-2286-6_20
Rashed, M.H., Hoque, T.S., Jahangir, M.M.R., Hashem M.A. (2021). Manganese as a Micronutrient in Agriculture: Crop Requirement and Management. Journal of Environmental Science and Natural Resources, 12(1-2), 225–242. https://doi.org/10.3329/jesnr.v12i1-2.52040
Martias, Hariyanto, B., Purnama, T., Nofiarli, Emilda, D., Hendri, Siregar, A.F., Kasno, A., Yuliati, S., Hernita, D., Arsana, I.G.K.D., Mejaya, M.J. (2021). Critical Level of Manganese in Soil and Leaves: It's Relationship to Fruit Quality of Mangosteen (Garcinia mangostana L.). Annual Research & Review in Biology. 36(9), 75–85. https://doi.org/10.9734/ARRB/2021/v36i930427
Mahdi Salih, M., Irzoqi, A. (2018). Synthesis and Characterization Complexes of Ni(II) that Contain Cyanoguanidine and Phosphines Ligands. Tikrit Journal of Pure Science, 23(1), 92–101. https://doi.org/10.25130/tjps.23.2018.014
Du, J., Liu, X., Zhang, J., Liu, Y., Zhu, E., Che, G., Jia, M. (2019). Facile Synthesis of a Polycatenane Compound Basedon Ag-triazole Complexes and Phosphomolybdic Acid for the Catalytic Epoxidation of Olefins with Molecular Oxygen. Catalysts, 9(7), 1–13. https://doi.org/10.3390/catal9070568
Nanayakkara, D., Prashantha, M.A.B., Fernando, T.L.D., Dissanayake, C.K., Karunarathna, B. (2023). Detection and quantification of dicyandiamide (DCD) adulteration in milk using infrared spectroscopy: A rapid and cost-effective screening approach. Food and Humanity, 1, 1472–1481. https://doi.org/10.1016/j.foohum.2023.10.013
Casali, L., Feiler, T., Heilmann, M., Braga, D., Emmerling, F., Grepioni, F. (2022). Too much water? Not enough? In situ monitoring of the mechanochemical reaction of copper salts with dicyandiamide. Cryst Eng Comm, 24, 1292–1298. https://doi.org/10.1039/D1CE01670A
Rwei S.P., Lin, Y.T., Yeh, S.K. (2014). A flame-retardant copper-clad laminate composite made of (metallocenebased cyclic olefin copolymer/glass) / cresol-novolak epoxy with low dielectricity. Textile Research Journal, 85(5), 524–534. https://doi.org/10.1177/0040517514548750
Atakan, R., Bical, A., Celebi, E., Ozcan, G., Soydan, N., Sezai Sarac, A. (2019). Development of a flameretardant chemical forfinishing of cotton, polyester, and CO/PET blends. Journal of Industrial Textiles, 49(2), 1–21. https://doi.org/10.1177/1528083718772303
Trivedi, M. K., Tallapragada, R. M., Branton, A. (2015). Characterization of Physical, Spectral and Thermal Properties of Biofield Treated 1,2,4-Triazole. Journal of Molecular Pharmaceutics & Organic Process Research, 3(2), 1–6. https://doi.org/10.4172/2329-9053.1000128
Voytenko, L.V., Kosmatiy, V.E., Kopilevich, V.A., Lavryk, R.V. (2012). Analytical Chemіstry: Manual for the Students of Institutions of Higher Education. National University of Life and Enviromental Sciences of Ukraine.
El-Naggar, М.A., Al-Rasheed, H.H, AL-khamis, S.A., El-Faham, A., Abu-Youssef, M.A.M., Haukka, M., Barakat, A., Sharaf, M.M., Soliman, S.M. (2023). Synthesis and X-ray Structure Analysis of the Polymeric [Ag2(4-Amino-4H-1,2,4-triazole)2(NO3)]n(NO3)n Adduct: Anticancer, and Antimicrobial Applications. Inorganics, 10(11) 395. https://doi.org/10.3390/inorganics11100395
John, C. Young, O’C. (2013). True Melting Point Determination. Chem. Educator, 18, 203–208. https://doi.org/10.1333/s00897132500a
Nijjer, S., Thonstad, J., Haarberg, G.M. (2000). Oxidation of manganese(II) and reduction of manganese dioxide in sulphuric acid. Electrochimica Acta, 46(2-3), 395–399. https://doi.org/10.1016/S0013-4686(00)00597-1
Warner, T.E., Bancells, M.M., Brilner Lund P., Lund, F.W., Ravnsbæk, D.B. (2019) On the thermal stability of manganese(II) sulfate and its reaction with zeolite A to form the sodalite Na6Mn2[Al6Si6O24](SO4)2. Journal of Solid State Chemistry, 277, 434–440. https://doi.org/10.1016/j.jssc.2019.06.038
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

