STUDY OF THE MECHANISMS OF THE INITIAL STAGES OF MnO2 FORMATION IN SULPHATE AND ACETATE ELECTROLYTES USING THE RRDE METHOD IN COMPARISON WITH THE RESULTS OF QUANTUM-CHEMICAL CALCULATIONS
DOI:
https://doi.org/10.15421/jchemtech.v33i2.329025Keywords:
Mangan dioxide; RRDE; quantum chemical calculations.Abstract
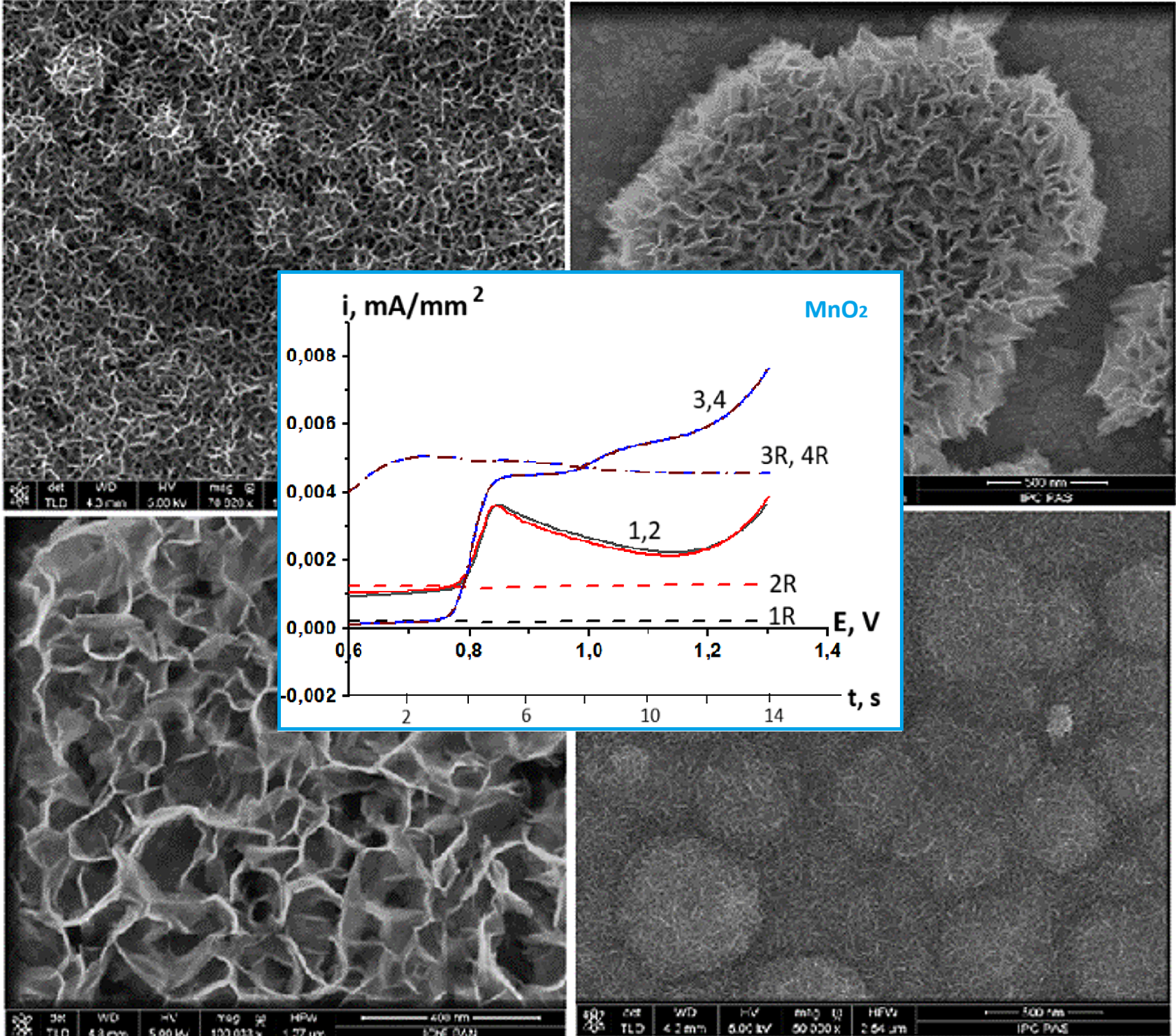
The formation of selective electrodes with high sensitivity for electrochemical biosensors is one of the most important tasks in the development of such devices. Therefore, understanding the mechanisms of electrochemical formation of MnO2 as an electrode material is an important factor in the strategy of coating synthesis for the determination of hydrogen peroxide in microquantities. In this article, for the first time, such a mechanism is analysed by comparing RRDE (rotating disk electrode with a ring) measurements with the results of quantum chemical calculations. The investigation was carried out in sulphate solutions with pH 1, pH 2 and pH 3.5. In sulfate electrolytes with pH 1 and pH 2, the mechanism of direct electron transfer has been fixed at the potentials for the initiation of the oxidation reaction of Mn2+ particles. The proposed mechanism of oxidation of divalent manganese by active oxygen-containing particles was not recorded. But we do not exclude their influence at more positive potentials. We note the important influence of hydrolysis on the process, which is seen in the difference in RRDE dependences at different pH of solutions. We assume that with an increase in pH to 3.5, Mn3+ hydrolyzed complexes attach to the surface and further transformations are associated with oxidation in a dense surface coating. The process occurring in the acetate electrolyte was also investigated. The RRDE method confirmed the assumption of the possibility of disproportionation of Mn3+ particles to Mn2+ and Mn4+ in the composition of acetate complexes. SEM photographs of MnO2 coatings obtained in the studied electrolytes illustrate that the nature of the electrolyte and the pH of the solution affect the structure and morphology of the resulting precipitate.
References
Sadeghi, M. H., Radmehr, S., Mohagheghzadeh, N., Fathi, J., Malekzadegan, Y., Moghadam, H. Z. (2025). Innovative electrochemical biosensors for tuberculosis detection. Clin. Chim. Acta., 574, 120327. https://doi.org/10.1016/j.cca.2025.120327.
Leote, R. J. B., Barsan, M. M., Sanz, C. G., Diculescu V. C. (2025). Electrochemical bienzymatic biosensor for pyruvate kinase activity evaluation and inhibitor screening. Talanta, 291, 127886. https://doi.org/10.1016/j.talanta.2025.127886.
Li, W., Gao, Y., Han, C., Luo, X., Zhao, J., Luo, H., Yang, J., Zhang, L. (2025). Electrochemical biosensor for point-of-care testing aqueous molecular hydrogen. Electrochim. Acta, 517, 145756. https://doi.org/10.1016/j.electacta.2025.145756.
Fontana-Escartín, A., Bertran, O., Alemán, C. (2024). Materials engineering in electrochemical biosensors: A review of cost-effective approaches to efficient biodetection. Mater. Today Commun., 41, 111030. https://doi.org/10.1016/j.mtcomm.2024.111030.
Zhu, H., Xu, G. (2025). Electrochemical biosensors for dopamine. Clin. Chim. Acta., 163, 108913. https://doi.org/10.1016/j.bioelechem.2025.108913.
Seipetdenova, S., Oladejo, T. O., Bekmurzayeva, A., Tan, K. L. C., Yang, M., Blanc, W., Tosi, D. (2025). Label-free multiplexed detection of diabetic retinopathy biomarkers using fiber optic biosensors: Towards lab-in-the-tear. Opt. Lasers Eng., 189, 108943. https://doi.org/10.1016/j.optlaseng.2025.108943.
Wang, X., Zhang, Y., Di, H., Qi, C., Xu, H., Lu, X., Shi, G., Cheng, S., Zhang, W. (2025). Detection of Salmonella in food by SG4MB/SRCA based colorimetric biosensor. J. Food Compos. Anal., 144, 107677. https://doi.org/10.1016/j.jfca.2025.107677.
Hirst, N. A., Hazelwood, L. D, Jayne, D. G., Millner, P. A. (2013) An amperometric lactate biosensor using H2O2 reduction via a Prussian Blue impregnated poly(ethyleneimine) surface on screen printed carbon electrodes to detect anastomotic leak and sepsis. Sensors & Actuators, B: Chemical, 186, 674–680. https://doi.org/10.1016/j.snb.2013.06.090.
Shi, Z., Wu, T., Feng, W., Hu, B., Yan, X., Zheng, X. (2024). Enhanced luminol chemiluminescence with oxidase-like properties of prussian blue/MXene nanocomposite without H2O2 for the sensitive detection of uric acid. Microchem J., 206, 111455. https://doi.org/10.1016/j.microc.2024.111455.
Rojas, D., Pelle, F. D., Del Carlo, M., d’Angelo, M., Dominguez-Benot, R., Cimini, A., Escarpa, A., Compagnone, D. (2018). Electrodeposited Prussian Blue on carbon black modified disposable electrodes for direct enzyme-free H2O2 sensing in a Parkinson’s disease in vitro model. Sensors & Actuators, B: Chemical, 275, 402-408. https://doi.org/10.1016/j.snb.2018.08.040.
Mahajana, A. P., Gaidhanea, H. M., Kondawarb, S. B. (2021). Comparative Study of Chronoamperometry of PANI/ZnO/Urease and PANI/MnO2/Urease Biosensors. IJARSCT, 12, 278-283. DOI:10.48175/IJARSCT-2389.
Xu, J.-J., Luo, X.-L., Du, Y., Chen, H.-Y. (2004). Application of MnO2 nanoparticles as an eliminator of ascorbate interference to amperometric glucose biosensors. Electrochem. commun., 6(11), 1169–1173. https://doi.org/10.1016/j.elecom.2004.09.015.
Nijjer, S., Thonstad, J., Haarberg, G.M. (2000). Oxidation of manganese(II) and reduction of manganese dioxide in sulphuric acid. Electrochim. Acta, 46(2–3), 395-399. https://doi.org/10.1016/S0013-4686(00)00597-1.
Dupont, M. F., Donne, S. W. (2014). Nucleation and Growth of Electrodeposited Manganese Dioxide for Electrochemical Capacitors. Electrochim. Acta, 120, 219–225. https://doi.org/10.1016/j.electacta.2013.12.014.
Begum, H., Ahmed, M. S., Jeon, S. (2019). δ-MnO2 nanoflowers on sulfonated graphene sheets for stable oxygen reduction and hydrogen evolution reaction. Electrochim. Acta, 296, 235-242. https://doi.org/10.1016/j.electacta.2018.11.073.
Fajardo, S., Ocón, P., Arranz, A., Rodríguez, J. L., Pastor, E. (2024). MnO2-modified ZIF-67 supported on doped reduced graphene oxide as highly active catalyst for the oxygen reduction reaction. J. Catal., 432, 115448. https://doi.org/10.1016/j.jcat.2024.115448.
Clarke, C. J., Browning, G. J., Donne, S. W. (2006). An RDE and RRDE study into the electrodeposition of manganese dioxide. Electrochim. Acta, 51(26), 5773–5784. https://doi.org/10.1016/j.electacta.2006.03.013.
Tsagareli, G., Makhatadze, S., Soselia, M., Maisuradze, N. (2022). Study of the initial stage of manganese dioxide electrodeposition on a rotating ring-disk electrode. J Indian Chem Soc., 99(6), 100499. https://doi.org/10.1016/j.jics.2022.100499.
Poltavets, V., Krawczyk, M., Maslak, G., Abraimova, O., Jönsson-Niedziółka, M. (2023). Formation of MnO2-coated ITO electrodes with high catalytic activity for enzymatic glucose detection. Dalton Trans., 52, P. 13769–13780. https://doi.org/10.1039/D3DT02199H.
Levich, V. G. (1962)/ Physicochemical Hydrodynamics. Prentice-Hall.
Frumkin, A., Nekrasov, L., Levich, B., Ivanov, Ju. (1959). Die anwendung der rotierenden scheibenelektrode mit einem ringe zur untersuchung von zwischenprodukten elektrochemischer reaktionen. J. Electroanal. Chem., 1(1), 84–90. https://doi.org/10.1016/0022-0728(59)80012-7.
Poltavets, V. V., Vargalyuk, V. F., Seredyuk, V. O., Shevchenko, L. V. (2018). [Mechanism of electrooxidation of Mn2+ ions]. J Chem Technol., 26(2), 1–11. (in Ukrainian)
Poltavets, V. V. (2019). [Electrochemical formation and properties of electrode materials based on MnO2]. (Candidate of Chemical Sciences dissertation), Dnipropetrovsk National University, Dnipropetrovsk, Ukraine (in Ukrainian).
Davies, D. (1969). Some aspects of the chemistry of manganese(III) in aqueous solution. Coord. Chem. Rev., 4(2), 199–224. https://doi.org/10.1016/S0010-8545(00)80086-7.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

