MASS TRANSFER DURING THE DISSOLUTION OF SODIUM TETRABORATE IN WATER INTENSIFIED BY MECHANICAL STIRRING
DOI:
https://doi.org/10.15421/jchemtech.v33i3.331092Keywords:
sodium tetraborate, borax, dissolution, agitation, diffusion, mass transferAbstract
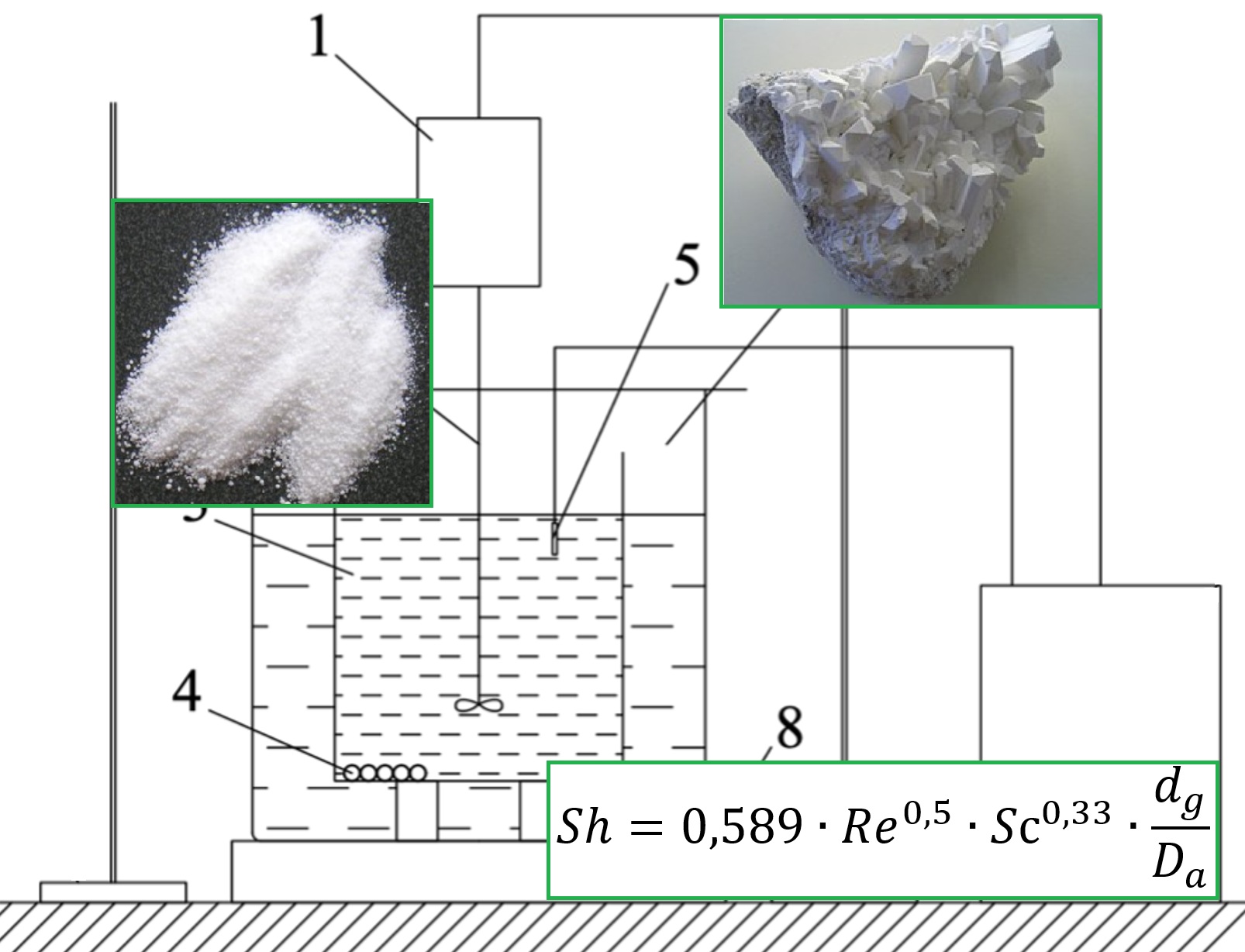
The process of mass transfer during the dissolution of sodium tetraborate granules in water was investigated at temperatures ranging from 293 K to 323 K, and with agitator rotation rates of 1.67 to 6.67 s-1. The study aimed to determine the dependency of the dissolution rate on the agitator rotation rate and water temperature. An analysis of the experimentally obtained results revealed that the most significant factor influencing the intensification of the dissolution process is the increase in the solution's temperature. The phenomena of external and internal diffusion during the dissolution of sodium tetraborate granules in water under agitation were also examined. A generalized criterion equation was applied to assess the mass transfer process, considering all the investigated factors. A comparison between experimental and theoretically calculated values showed that the maximum absolute relative error did not exceed 6 %. The obtained results are valuable for further research and potential applications in the chemical industry, pharmacology, and cosmetology.
References
Bernard, C. E., Harrass, M. C., Manning, M. J. (2010). Boric acid and inorganic borate pesticides. In R. Krieger (Ed.), Hayes’ Handbook of Pesticide Toxicology. Elsevier. https://doi.org/10.1016/B978-0-12-374367-1.00094-X
Gattey, D. (2008). Chemical-induced ocular side effects. In Frederick T. Fraunfelder, Frederick W. Fraunfelder, Wiley A. Chambers (Eds.), Clinical Ocular Toxicology. Elsevier. https://doi.org/10.1016/B978-1-4160-4673-8.10008-7
Schultz, T. P., Nicholas, D. D. (2004). Solid wood processing: Protection of wood against biodeterioration. In J. Burley et al. (Eds.), Encyclopedia of Forest Sciences. Elsevier. https://doi.org/10.1016/B0-12-145160-7/00048-X
Humnytskyi, Y., Symak, D., Nahurskyi, O. (2015). Dissolution of solids in a three-phase system formed by vacuuming. Scientific Works of Odessa National Academy of Food Technologies, 47(1), 130–133. (In Ukrainian).
Ahir, A. A., Mali, S. S., Hajare, A. A., Bhagwat, D. A., Patrekar, P. V. (2015). Pelletization technology: Methods and applications – A review. Research Journal of Pharmacy and Technology, 8(2), 131. https://doi.org/10.5958/0974-360X.2015.00023.2
Hörmann, T., Suzzi, D., Khinast, J. G. (2011). Mixing and dissolution processes of pharmaceutical bulk materials in stirred tanks: Experimental and numerical investigations. Industrial & Engineering Chemistry Research, 50(21), 12011–12025. https://doi.org/10.1021/ie2002523
Brian, P. L. T., Hales, H. B., Sherwood, T. K. (1969). Transport of heat and mass between liquids and spherical particles in an agitated tank. AIChE Journal, 15(5), 727–733. https://doi.org/10.1002/aic.690150518
Levins, D. M., Glastonbury, J. R. (1972). Application of Kolmogoroff’s theory to particle–liquid mass transfer in agitated vessels. Chemical Engineering Science, 27(3), 537–543. https://doi.org/10.1016/0009-2509(72)87009-X
Harriott, P. (1962). Mass transfer to particles: Part I. Suspended in agitated tanks. AIChE Journal, 8(1), 93–101. https://doi.org/10.1002/aic.690080122
Miller, D. N. (1974). Scale‐up of agitated vessels gas‐liquid mass transfer. AIChE Journal, 20(3), 445–453. https://doi.org/10.1002/aic.690200303
Nienow, A. W., Miles, D. (1978). The effect of impeller/tank configurations on fluid-particle mass transfer. The Chemical Engineering Journal, 15(1), 13–24. https://doi.org/10.1016/0300-9467(78)80033-1
Pangarkar, V. G., Yawalkar, A. A., Sharma, M. M., Beenackers, A. A. C. M. (2002). Particle−liquid mass transfer coefficient in two-/three-phase stirred tank reactors. Industrial & Engineering Chemistry Research, 41(17), 4141–4167. https://doi.org/10.1021/ie010933j
Boon-Long, S., Laguerie, C., Couderc, J. P. (1978). Mass transfer from suspended solids to a liquid in agitated vessels. Chemical Engineering Science, 33(7), 813–819. https://doi.org/10.1016/0009-2509(78)85170-7
Lal, P., Kumar, S., Upadhyay, S. N., & Upadhya, Y. D. (1988). Solid-liquid mass transfer in agitated Newtonian and non-Newtonian fluids. Industrial & Engineering Chemistry Research, 27(7), 1246–1259. https://doi.org/10.1021/ie00079a027
Dib, A., Makhloufi, L. (2006). Mass transfer correlation of simultaneous removal by cementation of nickel and cobalt from sulphate industrial solution containing copper. Chemical Engineering Journal, 123(1–2), 53–58. https://doi.org/10.1016/j.cej.2006.06.020
Bong, E. Y., Eshtiaghi, N., Wu, J., Parthasarathy, R. (2015). Optimum solids concentration for solids suspension and solid–liquid mass transfer in agitated vessels. Chemical Engineering Research and Design, 100, 148–156. https://doi.org/10.1016/j.cherd.2015.05.021
Nikhade, B. P., & Pangarkar, V. G. (2007). Theorem of corresponding hydrodynamic states for estimation of transport properties: Case study of mass transfer coefficient in stirred tank fitted with helical coil. Industrial & Engineering Chemistry Research, 46(10), 3095–3100. https://doi.org/10.1021/ie060385f
Winterbottom, M., Fishwick, R., & Stitt, H. (2003). Solid‐liquid mass transfer and hydrodynamics in the hydrogenation of 4‐nitrobenzoic acid. The Canadian Journal of Chemical Engineering, 81(3–4), 588–596. https://doi.org/10.1002/cjce.5450810334
Lee, T., Hou, H. J., Hsieh, H. Y., Su, Y. C., Wang, Y. W., Hsu, F. B. (2008). The prediction of the dissolution rate constant by mixing rules: The study of acetaminophen batches. Drug Development and Industrial Pharmacy, 34(5), 522–535. https://doi.org/10.1080/03639040701744194
Tschentscher, R., Spijkers, R. J. P., Nijhuis, T. A., van der Schaaf, J., Schouten, J. C. (2010). Liquid−solid mass transfer in agitated slurry reactors and rotating solid foam reactors. Industrial & Engineering Chemistry Research, 49(21), 10758–10766. https://doi.org/10.1021/ie100385n
El‐Naggar, M. A., Abdel‐Aziz, M. H., Zatout, A. A., & Sedahmed, G. H. (2014). Liquid‐solid mass transfer behavior of a stirred‐tank reactor with a fixed bed at its bottom. Chemical Engineering & Technology, 37(9), 1525–1531. https://doi.org/10.1002/ceat.201300689
Carletti, C., de Blasio, C., Mäkilä, E., Salonen, J., Westerlund, T. (2015). Optimization of a wet flue gas desulfurization scrubber through mathematical modeling of limestone dissolution experiments. Industrial & Engineering Chemistry Research, 54(40), 9783–9797. https://doi.org/10.1021/acs.iecr.5b02691
Symak, D., Luta O. (2015). Nestatsionarnyi protses rozchynennia sharu zernystoho materialu. Khimiia, tekhnolohiia rechovyn ta yikh zastosuvannia, 812, 308–312.
Symak, D., Gumnitsky, J., Atamaniuk, V., Nagurskyy, O. (2017). Investigation of physical dissolution of benzoic acid polydisperse mixture. Chemistry & Chemical Technology, 11(4), 469–474. https://doi.org/10.23939/chcht11.04.469
Symak, D., Atamanyuk, V., Gumnytskyy, Y. (2015). Analysis of dissolution kinetics based on the local isotropic turbulence theory. Chemistry & Chemical Technology, 9(4), 493–496. https://doi.org/10.23939/chcht09.04.493
Danyliuk, O. M., Atamaniuk, V. M., Hnativ, Z. Ya. (2018). The influence of mixing solid particles on the kinetic of benzoic acid dissociation during the pneumatic mixing of solution. Scientific Bulletin of UNFU, 28(7), 92–96. https://doi.org/10.15421/40280720
Patmonoaji, A., Tahta, M. A., Tuasikal, J. A., She, Y., Hu, Y., Suekane, T. (2023). Dissolution mass transfer of trapped gases in porous media: A correlation of Sherwood, Reynolds, and Schmidt numbers. International Journal of Heat and Mass Transfer, 205, 123860. https://doi.org/10.1016/j.ijheatmasstransfer.2023.123860
Joshi, S.S., Dalvi, V. H., Vitankar, V. S., Joshi, A. J., Joshi, J.B. (2023). Novel correlation for the solid–liquid mass transfer coefficient in stirred tanks developed by interpreting machine learning models trained on literature data. Industrial & Engineering Chemistry Research, 62(46), 19920–19935. https://doi.org/10.1021/acs.iecr.3c02442
Kuzyk, O., Atamaniuk, V., Gumnitsky, Y. (2024). Mass transfer during boric acid dissolution. Chemistry & Chemical Technology, 18(3), 393–400. https://doi.org/10.23939/chcht18.03.393
Rumble, J. R., Brunno, T. J., Doa, M. J. (Eds.). (2023). CRC Handbook of Chemistry and Physics: A Ready-reference Book of Chemical and Physical Data (104th ed.). CRC Press.
Miyabe, K., Isogai, R. (2011). Estimation of molecular diffusivity in liquid phase systems by the Wilke–Chang equation. Journal of Chromatography A, 1218(38), 6639–6645. https://doi.org/10.1016/j.chroma.2011.07.018
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

